RECIST 1.1 - and more
Response Evaluation Criteria In Solid Tumors
Fokko Smits, Martijn Dirksen and Ivo Schoots
Radiology Department of the Erasmus MC in Rotterdam and the Isala hospital in Zwolle, the Netherlands
Publicationdate
RECIST is a standard way to measure the response of a tumor to treatment.
It provides objective criteria to determine whether a tumor disappears, shrinks, stays the same or gets bigger.
This is called complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD).
In this article we describe the five easy steps to follow when you want to use RECIST 1.1.
Recist 1.1 is not used for lymphoma, GIST during Glivec therapy, HCC and malignant brain tumors.
Other criteria have been published for these tumors.
In addition a brief overview is given of variations of RECIST and some other response systems.
Introduction
RECIST is a standard way to measure the response of a tumor to treatment.
If a study is eligible, choose target lesions (max 5) that are easy to measure and calculate the sum of the longest diameters (SLD).
Identify non-target lesions like ascites or pleural fluid that are not suited for exact measurements, but that can be followed.
In the follow up study compare these lesions and determine the response.
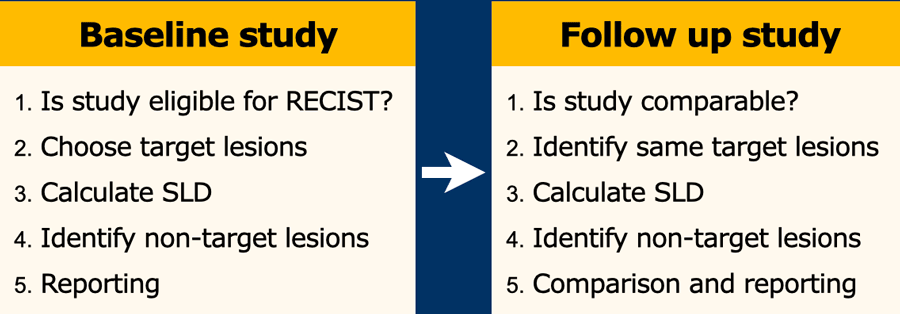
Baseline Study - five easy steps
1. Is the study eligible for RECIST?
Radiographic studies that may be used:
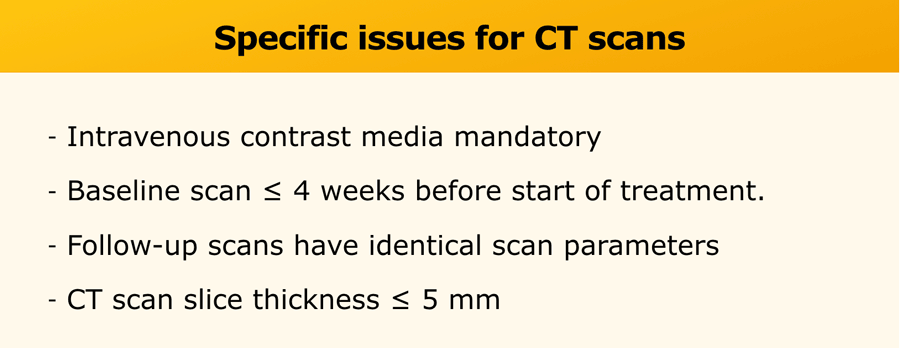
- CT is the preferred imaging modality. See table for specific issues.
- MRI may be used in some instances. Use the same criteria as for CT.
MR acquisition parameters should be specified and optimised.
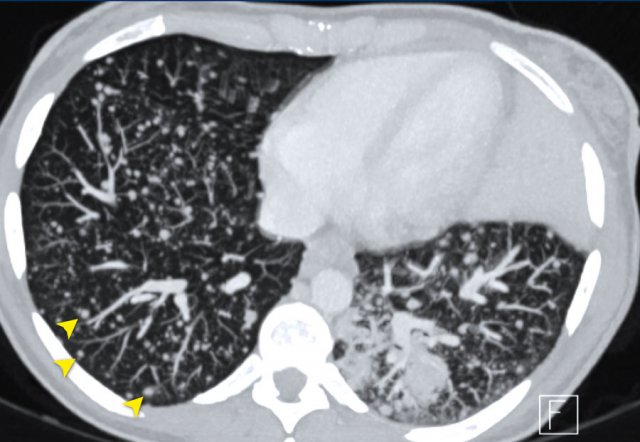
The parameters should be consistent during the trial. - Chest X-ray is used if lesions are clearly defined and surrounded by aerated lung.
The minimal size of target lesions must be ≥ 20mm.
Ultrasound is not used for RECIST due to operator dependency.
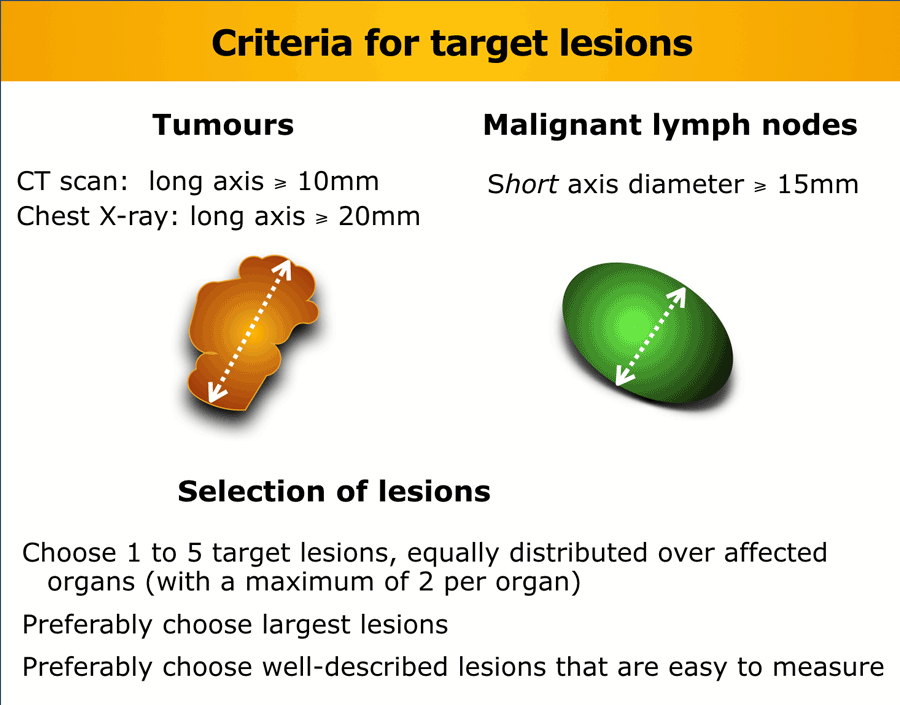
2. Choose “target lesions”
Tumors
Choose preferably large well-described lesions to measure with a maximum of two per organ and a maximum of five for the whole study.
Lymph nodes
Lymph nodes can be used as target lesions provided that the maximum short axis diameter exceeds 15 mm.
Nodes <10 mm are regarded as normal, while nodes 10-15mm are regarded as pathologic, but not suited for target lesions. They can be used as non-target lesions.
Not target lesions
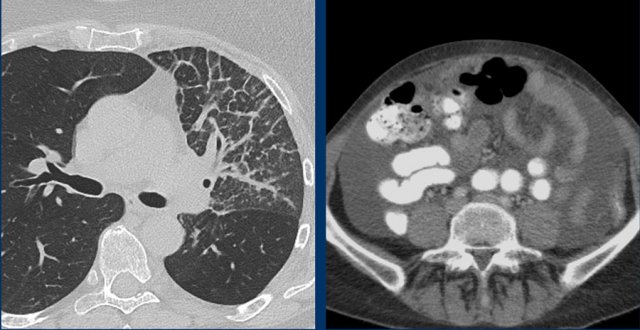
- Non-measurable lesions like leptomeningeal disease, ascites, effusions, inflammatory breast disease, and lymphangitic involvement of skin or lung.
- Lesions located in an area, that has been subjected to loco-regional therapy.
- Tumors that have other criteria for follow up like Lymphoma or GIST tumors during Glives therapy, HCC, malignant brain tumors.
- Bone lesions without soft tissue component.
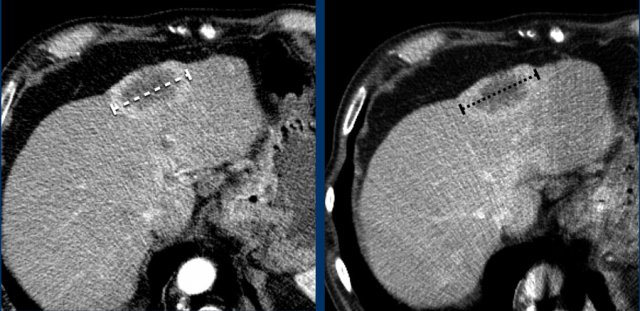
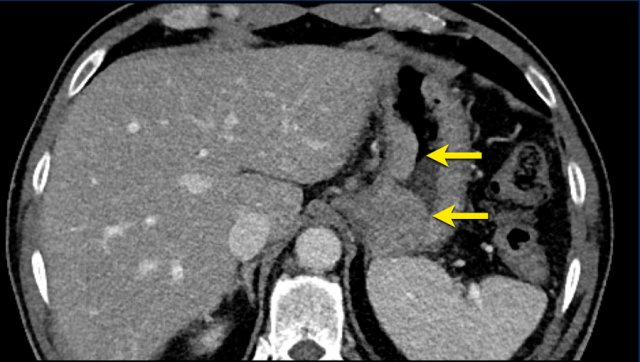
Measurement includes hypervascular rim
When a lesion has a hypervascular rim, this is included in measuring the longest diameter, because it represent viable tumor tissue.
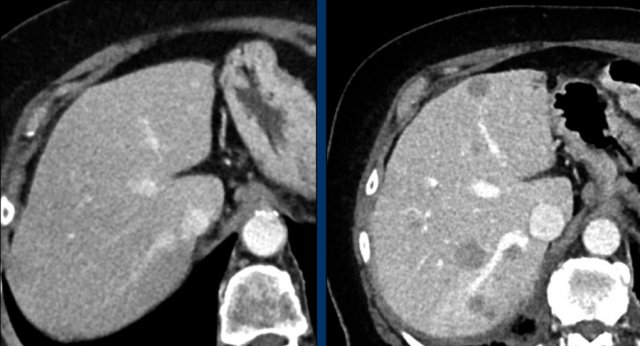
The CT images in the arterial and portovenous phase of a 71-year-old male show liver metastases of a neuro-endocrine tumour of the esophagus.
Note that the hypervascular rim is better appreciated in the arterial phase.
The large hypervascular rim is included in the measurement of the largest diameter.
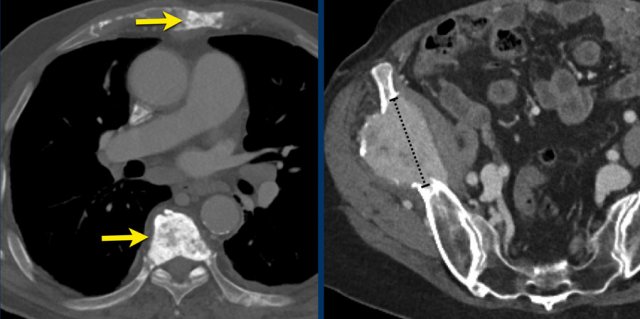
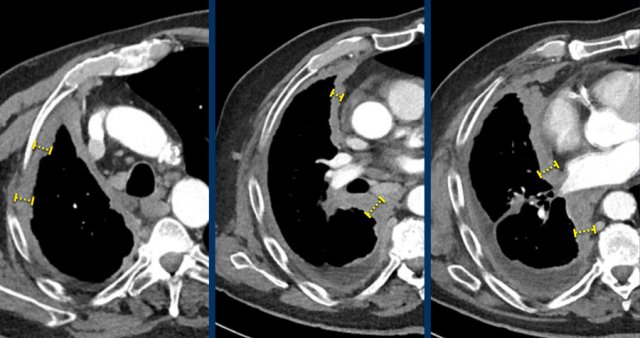 Measurements of the tumour thickness perpendicular to the chest wall or mediastinum in two positions at three different levels.
Measurements of the tumour thickness perpendicular to the chest wall or mediastinum in two positions at three different levels.
Mesothelioma
An exception to measuring the longest diameter is in patients with malignant pleural mesothelioma.
The non-spherical growth pattern in this disease makes reproducible long axis measurements difficult.
Therefore not the longest diameter, but the tumour thickness perpendicular to the chest wall is used.
This measurement has a good correlation with outcome.
CT images in a 63-year-old male with malignant pleural mesothelioma.
Measurements according to the modified RECIST 1.1 for malignant pleural mesothelioma with measurement of the tumour thickness perpendicular to the chest wall or mediastinum in two positions at three different levels, preferably in the upper thorax.
Example of non-measurable lesions in a patient with lymphangitis carcinomatosa and another patient with ascites.
These findings are used as non-target lesions and in the follow up their presence or absence are determined.
Bone metastases
During follow-up bone metastases quite often change in appearance while the size remains the same, therefore they are generally considered non-measurable lesions.
According to RECIST 1.1. only lytic or mixed lytic-blastic bone metastases with identifiable soft tissue component can be considered as measurable lesions if they meet the measurability criteria.
The left CT image is of a 80-year-old male with osteoblastic bone metastases of a non-small-cell lung carcinoma.
This lesion is not suitable for measurement.
The CT image on the right is of a 69-year-old female with an osteolytic metastasis in the right iliac bone.
This metastasis is suitable for measurement and can be used as target lesion.
Sometimes the largest lesion is not the most suitable for reproducible repeated measurements.
This CT image is of a 61-year-old male with gastric cancer and lymph node metastases.
There is a large lobulated mobile gastric tumor.
We can assume, that on a follow up examination it can not be reproduced in the same way.
Therefore this mobile tumor is not suitable as target lesion, but can be used as non-target lesion.
Continue with next image...
At a lower level there is an enlarged lymph node which is more suitable to be used a target lesion (arrow).
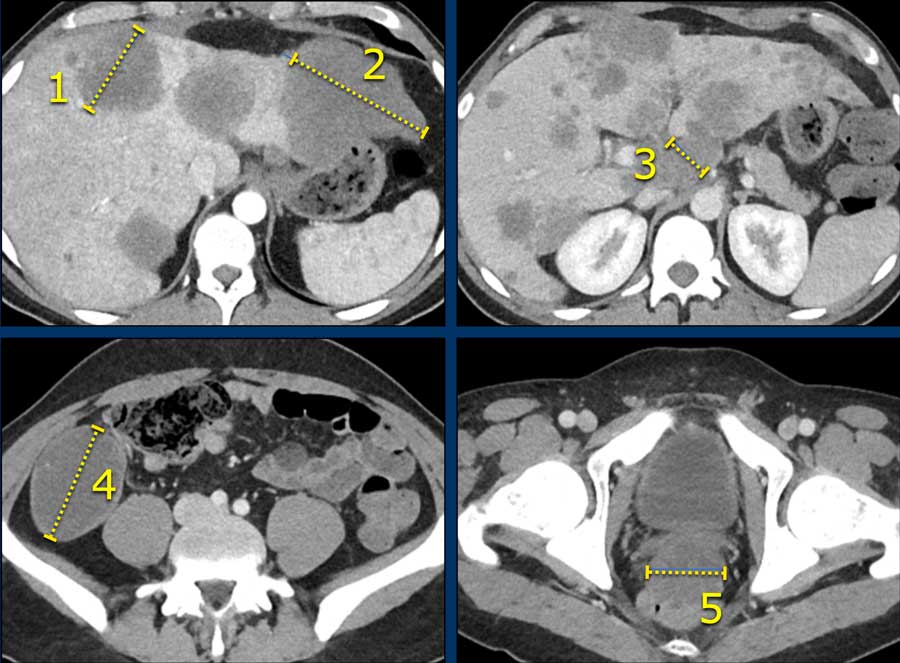
3. Calculate Sum of Longest Diameters
Here an example of 5 target lesions in a 28-year-old male with a neuroendocrine carcinoma of the appendix with liver, lymphogenic and peritoneal metastases.
4. Identify non-target lesions
What are “non-target” lesions”?
Non-target lesions are all other disease related lesions that do not meet the criteria for a target lesion like pleural fluid, ascites and miliary lung metastases, or those lesions that are supernumerary because the maximum number of 5 target lesions had been reached.
You do not measure these lesions.
Just mention them in the report and in the follow up look for their presence or absence.
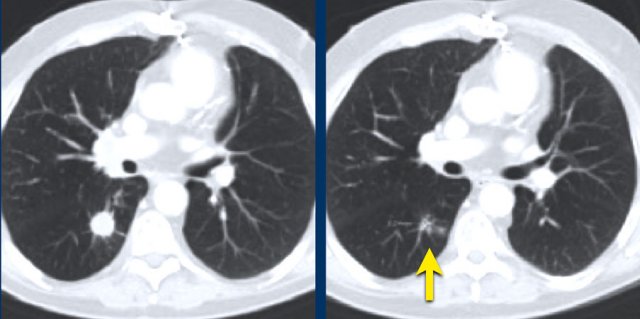
The CT image with maximum intensity projection of a 34-year-old female with miliary lung metastases of ovarian cancer.
The metastases cannot be used as target lesions because they are too small.
They can be used as non-target lesions and can be mentioned as a single item in the radiology report.
5. Report
The baseline radiology report should contain the following elements:
- Modality and parameters.
- Description of the target lesions. Each with localization, table position and size.
- Description of non-target lesions.
- Other incidental relevant findings.
- Conclusion with number of target lesions and their localization and clinically important incidental findings.
Follow up study
Is the study technically comparable to previous?
For follow-up studies, the same imaging modality should be used as for the baseline study and identical imaging parameters should be used, like slice-thickness, contrast protocol etc.
Identify same target lesions
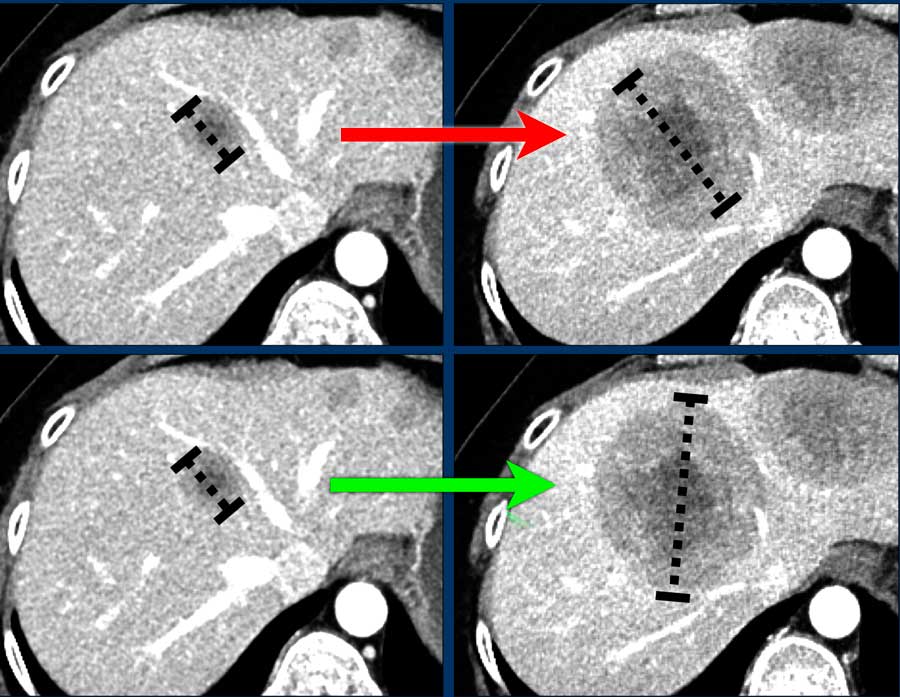
Orientation
If the orientation of longest diameter varies during follow-up, measure the longest diameter (fig).
Do's
- Do consider “new” lesions in an area of the body that was not imaged during baseline (for example brain metastases) as truly “new”, thereby
forcing overall response to progressive disease. - Do measure the short axis of mesothelioma.
Dont's
- Don't change target lesions or non-target lesions during follow-up studies: “once a target lesion, always a target lesion”
- Don’t change measurements on previous studies
- Don't measure lesions after treatment by RF ablation or cryoablation, because the ablated area is often larger than the original tumor.
- Don't regard “new” sclerotic bone lesions as progressive disease as they may represent filling-in of previously lytic lesions that were not detected at baseline.
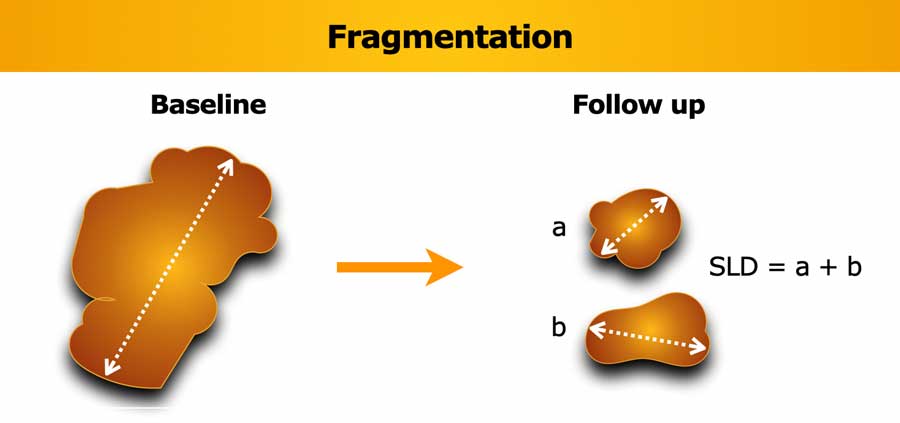
Fragmentation
If the lesion breaks into separate fragments between baseline and follow-up, the sum of longest diameters (SLD) of the fragments should be calculated.
Vice versa if lesions coalesce, then measure the longest diameter of the merged lesion only.
Obviously, the short axis diameter is measured in lymph nodes.
Too small to measure
If a target lesion has disappeared record a measurement of 0 mm.
If the lesion is too small to measure assign a default value of 5 mm to prevent false responses (derived from the 5 mm CT slice thickness).
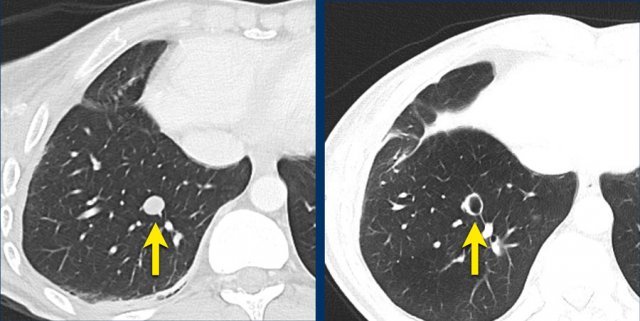
The images are of a patient with a primary lung tumour.
At baseline, the longest diameter is well above 10 mm, therefore this was assigned as a target lesion.
During follow-up the long-axis diameter dropped below 10 mm, which is the lower limit for considering a lesion as target lesion.
However, since this is a follow-up measurement, the target lesion still counts up to the sum of the diameters (SLD) and a default value of 5mm was assigned.
Cavitating lesions
Cavitation can occur during treatment.
Cavitating lesions should be continuously measured in their longest diameter.
A different assessment can be provided if the sum of diameters does not adequately correspond to the patients response assessment.
These CT images are of a 32-year-old male with a pulmonary metastasis of a malignant peripheral nerve sheath tumour.
Cavitation occured after treatment with pazopanib, but the size remained the same.
Although the size remains the same, a remark can be made in the report, that the actual tumorvolume has decreased.
Calculate SLD
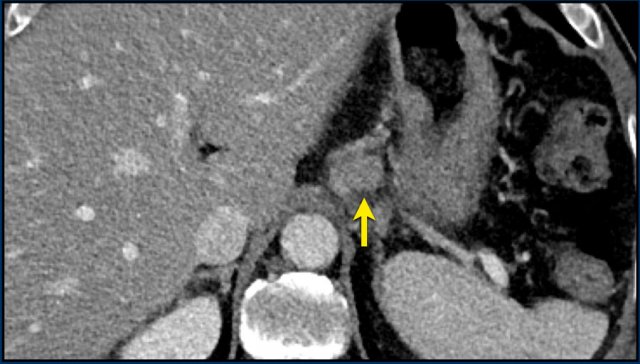
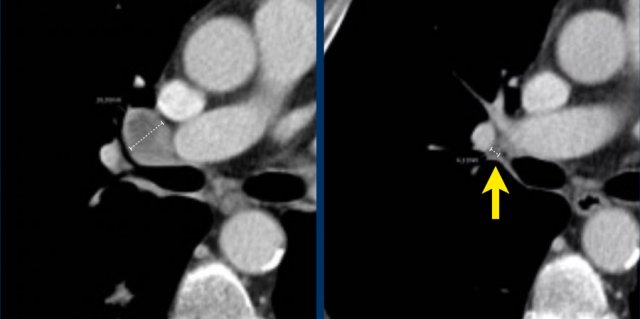
If during follow-up the short-axis diameter of a lymph node drops below 15 mm, the measurements are continued and still count for calculation of the SLD.
In this case the baseline short axis diameter is 18 mm.
This was a target lesion.
At follow-up, the short-axis diameter dropped below 15 mm. However, the measurements are continued, and the 4 mm diameter counts up for the SLD.
When lymph nodes decrease to a normal size (<10 mm), they still have to be included in the sum of the target lesions.
This means that whenever the lymph nodes enlarge again, you will not overstate the progression, but also that complete remission is possible even when the sum of the diameters is not zero.
Progression of non-target lesions
CT images in a 61-year-old male with melanoma during treatment.
At baseline the inguinal lymph nodes were to small to be used as target lesions and were regarded as non-target lesions.
At follow up there is unequivocal progression of the lymphogenic metastases.
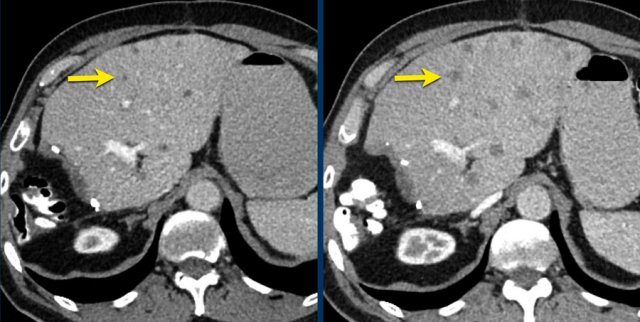 CT images in a 61-year-old male with unequivocal progression of non-target lymphogenic metastases of melanoma during treatment.
CT images in a 61-year-old male with unequivocal progression of non-target lymphogenic metastases of melanoma during treatment.
CT images in a 73-year-old male with progressive liver metastases of colorectal carcinoma.
This is anothe example of progression of non-target lesions.
Even if there is partial response or even disappearance of other lesions, this is still progressive disease.
New lesions
Any new lesion means progressive disease.
CT-images in a 81-year-old female with endometrial carcinoma and occurrence of new liver metastases during treatment with chemotherapy.
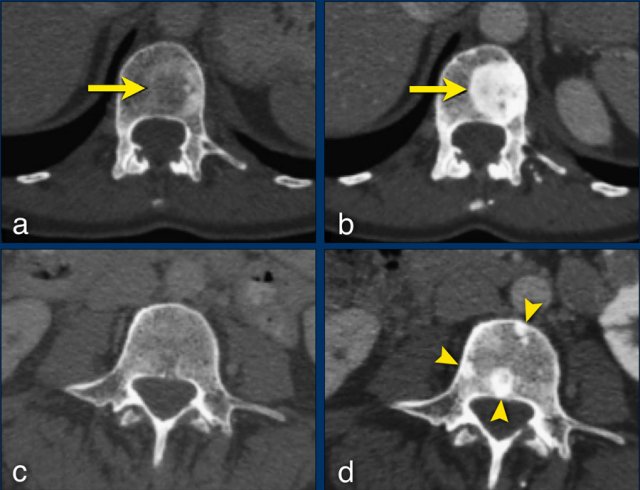 The sclerotic bone lesions in b and d are not new metastases but an osteoblastic reaction. Courtesy Els van Persijn van Meerten.
The sclerotic bone lesions in b and d are not new metastases but an osteoblastic reaction. Courtesy Els van Persijn van Meerten.
Any new lesion means progressive disease, but not every newly detected lesion is always a true new lesion.
In osteolytic bone metastases it can be difficult to determine if a sclerotic lesion that is detecting during follow up is truly a new lesion.
The CT images are of a 50-year-old female with bone metastases of a breast carcinoma.
At baseline (a), there is an osteolytic lesion in a thoracic vertebral body (arrow).
After chemotherapy, the thoracic osseous lesion has not changed in size, but has become completely osteoblastic (arrow in b), representing a good response.
In the lumbar vertebra no visible metastases were seen in the baseline scan (c).
The ‘new’ sclerotic lesions in the lumbar vertebra (arrowheads in d), are considered to be small osteolytic metastases that the baseline CT failed to identify.
They became visible due to the osteoblastic reaction.
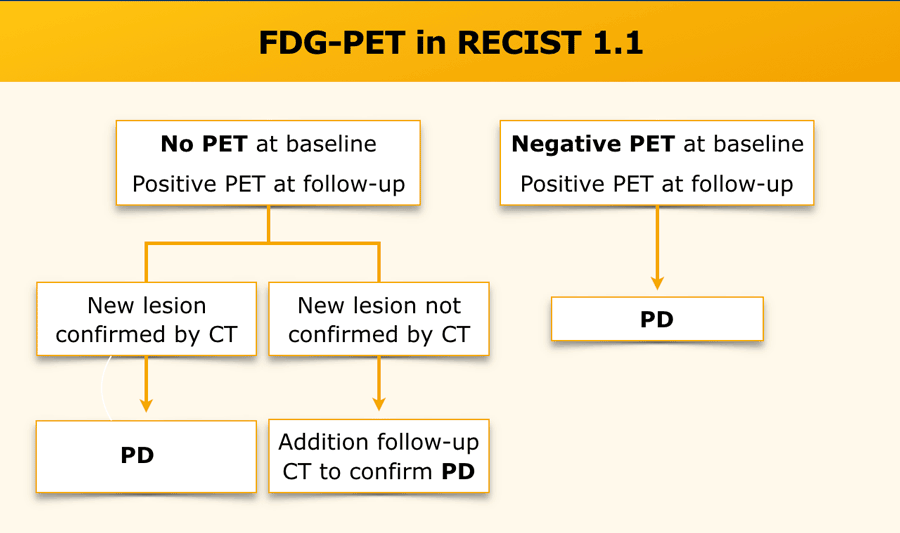
PET
FDG-PET can be complementary to diagnostic CT imaging in assessment of disease progression, especially in the case of a possible new lesion.
A positive FDG-PET at follow-up, with a negative FDG-PET at baseline, is a sign of progressive disease based on the new lesion.
Without an FDG-PET examination at baseline, findings are dependent on current and previous CT findings.
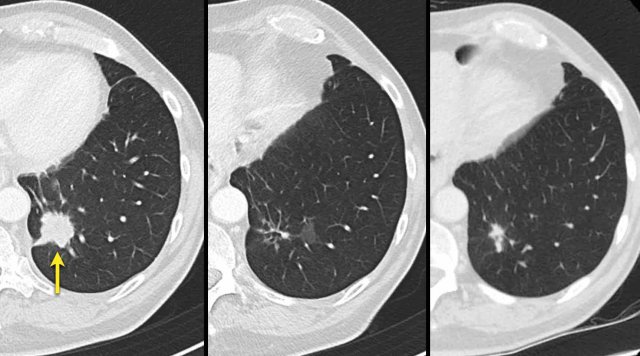 CT images in a 72-year-old male with recurrent esophageal carcinoma and a re-appearing lung metastasis.
CT images in a 72-year-old male with recurrent esophageal carcinoma and a re-appearing lung metastasis.
Reappearance of lesions
Disappearance and subsequent reappearance of a lesion in follow-up examinations should continue to be measured.
Depending on the disease status, a reappearing lesion can be considered either progressive disease, when the previous examination showed complete response or otherwise the maximal diameter should be added to the SLD for a calculated response.
The rationale is that most lesions do not disappear but are under the detection level of the employed imaging modality.
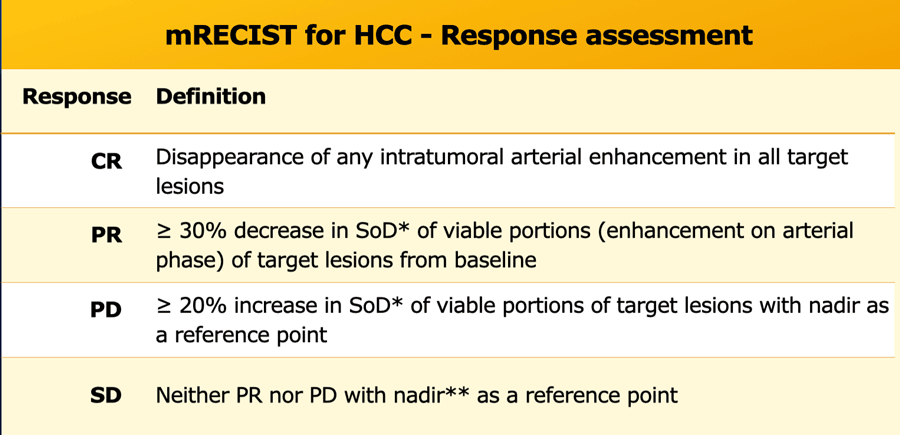
Response categories
To determine the response of a tumor in the follow up, we have to look at the target- and non-target lesions and look for any new lesions.
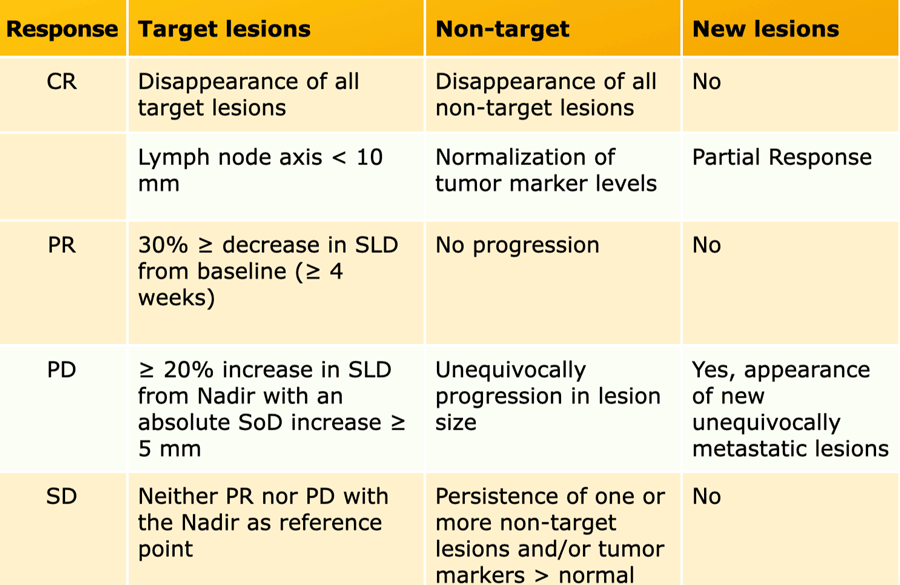
Progressive Disease (PD)
- ≥ 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on the study. This is called the Nadir. This includes the baseline sum if that is the smallest on the study.
- In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm.
- The appearance of one or more new lesions is always considered progression.
- Unequivocal progression of non-target lesions is also considered progression
Stable Disease (SD)
- Neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease, taking as reference the smallest sum of length diameters (SLD) while on the study.
Partial Response (PR)
- At least a 30% decrease in the sum of length diameters (SLD) of target lesions, as compared to baseline sum diameters.
Complete Response (CR)
- Disappearance of all target and non-target lesions.
- Any pathological lymph nodes must have reduction in short axis to <10 mm.
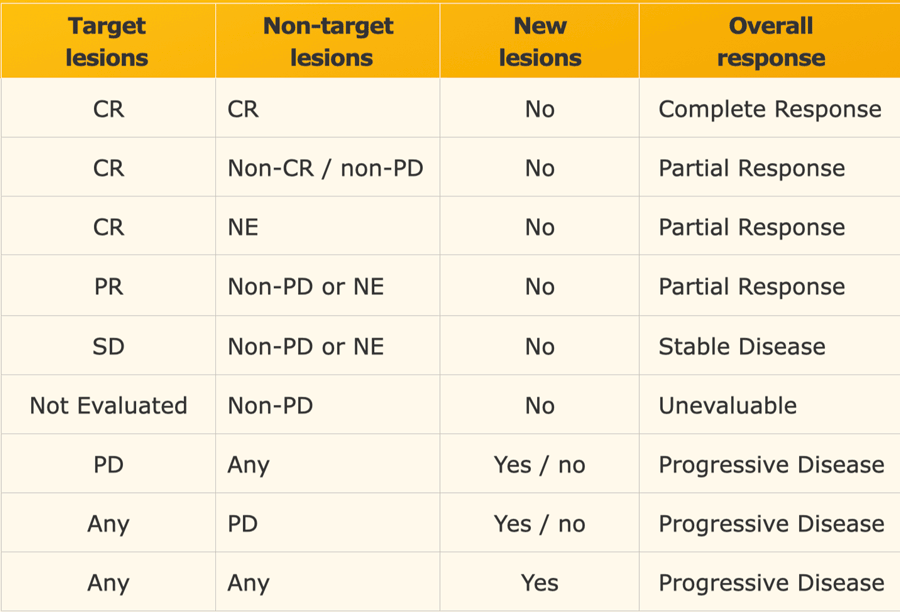
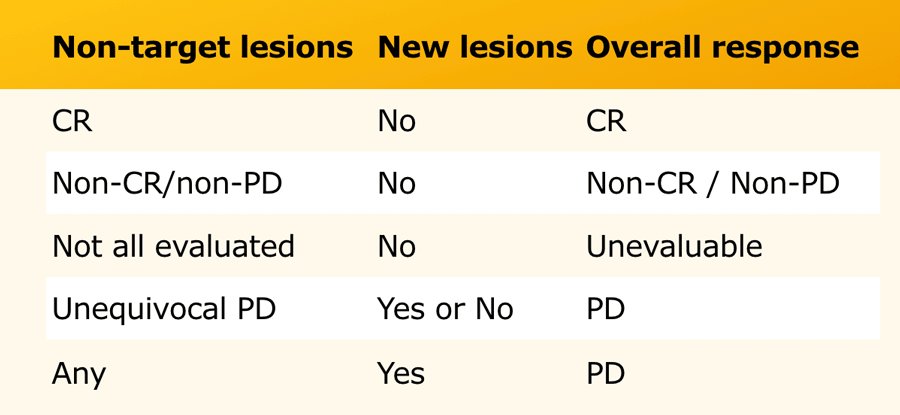
Response in target lesions with or without non-target disease
The overall response is based on the response of all tumor related findings (table).
For instance any progression ( >20% increase) of target or non-target lesions or any new lesion means progressive disease no matter how the other lesions reacted.
When tumor markers are initially elevated, they must normalize before considering complete response when all lesions have disappeared.
Response in only non-target lesions
In some patients there are no suitable target lesions that can be measured and there are only non-target lesions.
In these cases you have to make an estimation of the response (table).
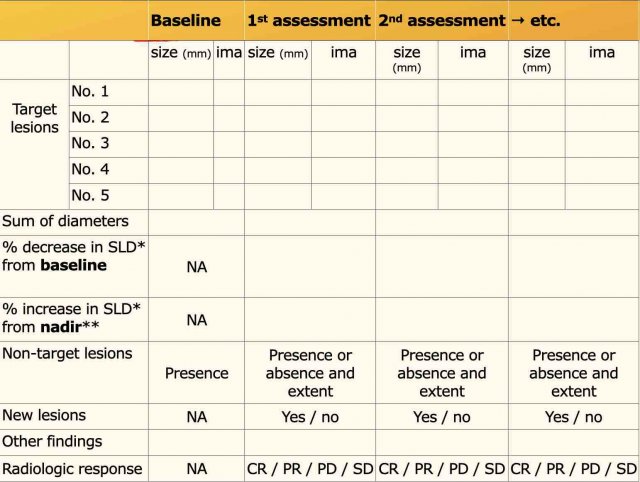
Radiology report
The radiology report of each follow-up study should contain the following elements:
Report
Modality and parameters
Description of target lesions with localization, table position and size
Description of non-target lesions and comparison: unchanged, decreased or increased in size.
New lesions?
Incidental relevant findings
Conclusion
Number of target lesions and their localization, overall impression of non-target lesions, clinically important incidental findings.
The table shows an example of a follow up report.
Calculation of SLD and assigning response categories by the radiologist depend on local agreement with the oncologist.
Quite commonly, only measurements of target disease and presence and extent of non-target disease are reported with an impression of the overall tumor response mentioned in the report’s conclusion.
Other Response systems
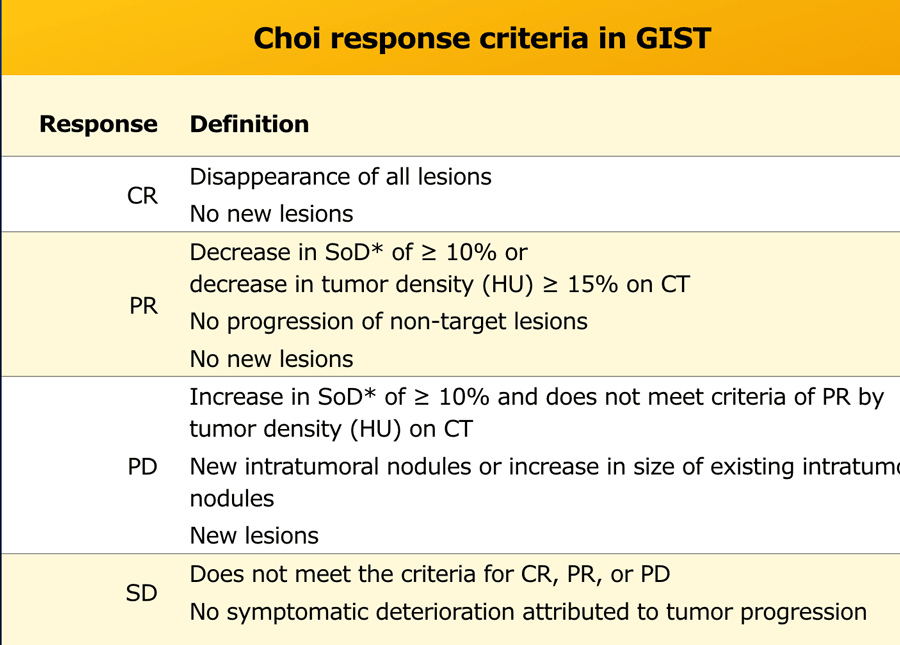
Choi criteria for GIST
The Choi criteria are based on RECIST and developed for The Choi criteria are based on RECIST and developed for assessing treatment response in patients with gastrointestinal stromal tumors (GIST) treated with imatinib (3).
Usually decrease in tumour size occurs in the course of treatment, however this doesn’t necessarily reflect tumour response.
Sometimes tumour size can increase due to internal hemorrhage, necrosis or myxoid degeneration.
Major difference of Choi response criteria in comparison to RECIST 1.1 is the introduction of tumour attenuation as an additional response parameter.
Reduction in tumor size is usually minimal in the early posttreatment response assessment, while significant changes in internal characteristics like tumour attenuation, nodularity, and number of vessels will occur.
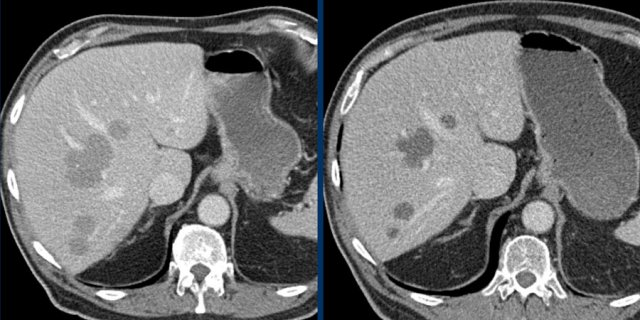
The CT images of a 82-year-old male show liver metastases of a GIST at baseline (arrowheads).
All metastases decrease somewhat in size after treatment with imatinib, but the most remarkable difference is a decrease in density.
This is considered to be a good response according to the Choi criteria.
Before the introduction of the Choi response criteria, recurrent or progressive disease used to be determined by an increase in tumour size or identification of new locations of disease.
Although increase in tumour size remains an important parameter for evaluating disease response, recurrent disease can occur within the treated tumor without increasing tumor size.
The CT images in a 66-year-old male show liver metastases of a GIST at diagnosis (a).
At 3 months after treatment with imatinib there is a good response (b).
At a follow up scan at 1 year there is a recurrence (arrow in c).
At a follow-up after 2nd line treatment with sunitinib there is still tumor progression, but the size remains the same (d).
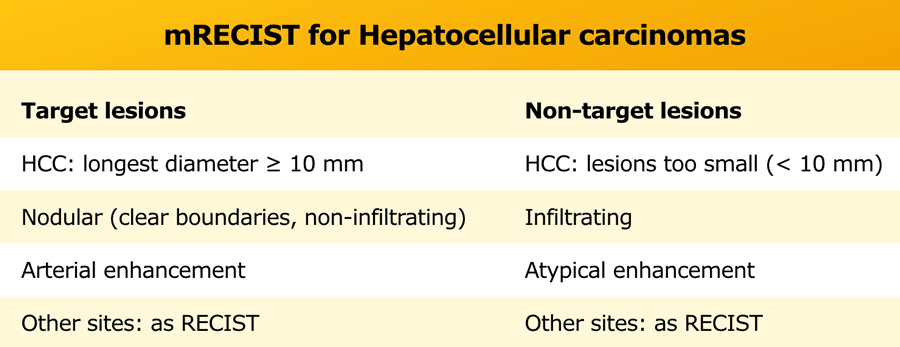
mRECIST for Hepatocellular carcinomas
Tumour response assessment based on changes in size alone can be deceptive when applied to hepatocellular carcinoma (HCC) treated with targeted or interventional therapies.
In 2000 the European Association for the Study of the liver (EASL) assembled an expert panel on HCC which suggested that the optimal method for treatment response assessment in HCC should be the estimation of viable tumour with contrast enhanced imaging.
These new criteria were based on RECIST 1.1 and referred to as modified RECIST (mRECIST) with the most important feature of defining viable tumour as uptake of contrast agent during arterial phase dynamic imaging on CT or MRI.
mRECIST is only used for typical lesions showing intratumoral arterial enhancement, while RECIST 1.1 is applied for atypical enhancing lesions and extrahepatic disease.
The table shows extra recommendations for the assessment of tumor response in non-target lesions in patients with HCC and cirrhosis.
Measurement rules in assessing response:
- Measurement of viable tumor on CT or MRI in the arterial phase.
- The same plane should be used to measure the longest diameter, but not necessarily the same level or measurement direction.
- Measurement should avoid any major intervening areas of necrosis.
Rules of progression:
- A newly detected liver nodule will be categorized as HCC (PD) when size >1 cm and typical enhancing pattern (hypervascularization in arterial phase with washout in portovenous or late phase).
- New liver nodules >1 cm with atypical enhancement patters can be diagnosed as HCC (PD) when >/= 1 cm interval growth in subsequent CT or MRI.
- Progressive disease will be determined in retrospect at the time it was first detected by imaging.
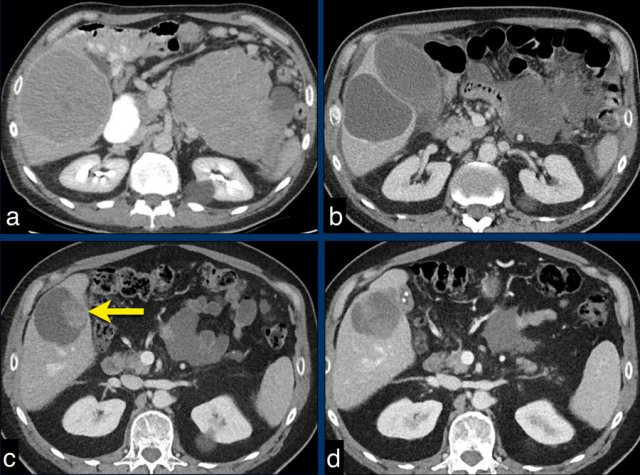
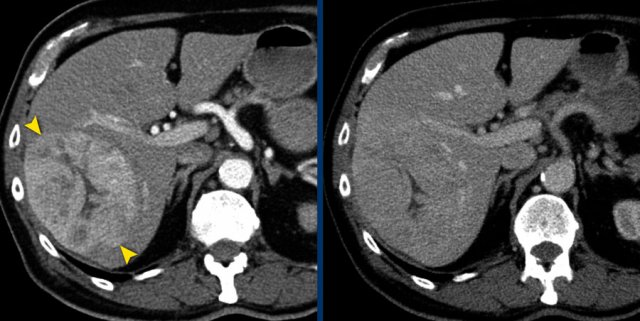
The CT image of the liver of a 96-year-old male with HCC in the late arterial phase shows a hypervascular tumour in the right liver lobe (arrowheads).
The tumor is well delineated from the surrounding parenchyma.
In the portal-venous phase the HCC is hardly distinguishable from the liver parenchyma due to early washout of contrast.
This HCC is suitable for mRECIST.
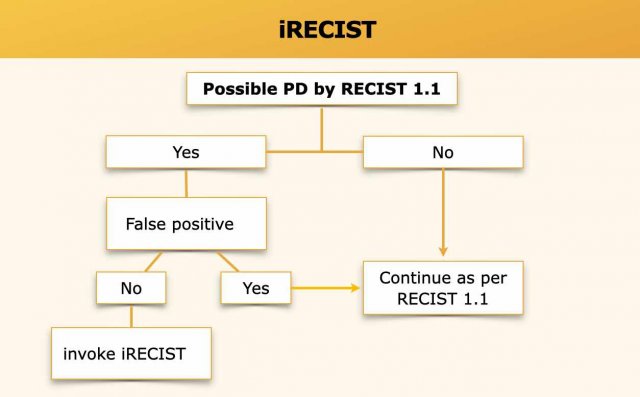
iRECIST for immune therapy
iRECIST represents a modified RECIST 1.1 for immune-based therapeutics.
The immunotherapeutic agents induce a different tumour response in comparison to standard chemotherapeutic agents.
The new mechanism of actions of these drugs, with immune and T-cell activation, can cause uncommon patterns of tumour response that look like tumour flare, termed pseudoprogression (4).
Pseudoprogression has been described in non-small cell lung carcinoma, melanoma and renal cell carcinoma.
These patients meet the criteria for disease progression according to traditional response criteria like RECIST, but can demonstrate late and durable responses.
The main criteria to objectively evaluate tumour response are mostly unchanged from RECIST 1.1.
The most important change is to identify true disease progression, which is defined as subsequent increase in tumour burden after an examination with unconfirmed progressive disease.
 *LDi = longest diameter, **SPD = the sum of the product of perpendicular diameters for multiple lesions
*LDi = longest diameter, **SPD = the sum of the product of perpendicular diameters for multiple lesions
Lugano Classification of malignant lymphoma
The Lugano classification incorporates PET-CT for initial evaluation, staging and response assessment of malignant lymphoma.
Contrast enhanced CT is used for staging low FDG-avid lymphomas or for more accurate measurements of nodal size in clinical trials, to discriminate between bowel and lymphadenopathy, assessing compression or thrombosis of vessels and for radiation planning.
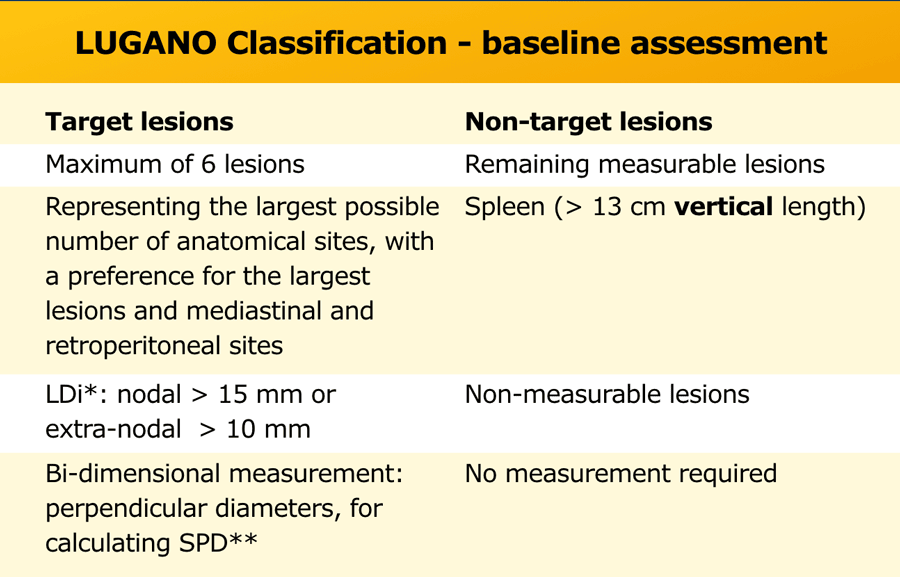
Measurable nodes must have a longest diameter (LDi) greater than 1.5 cm and measurable extranodal lesions must have a LDi greater than 1.0 cm.
Bi-dimensional measurement of perpendicular diameters are noted for calculating the product of perpendicular diameters (PPD) and the sum of the product of perpendicular diameters for multiple lesions (SPD).
Non-target disease includes the remaining measurable lesions (nodal and extranodal), spleen (> 13 cm vertical length) and non-measurable lesions (eg, smaller lesions, pleural and pericardial effusion, ascites).
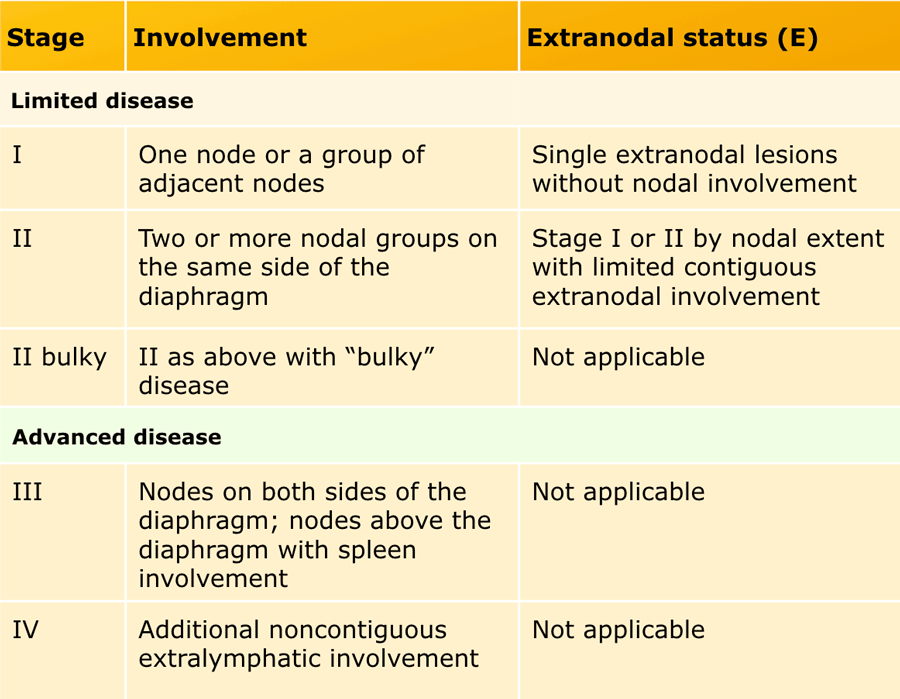
Modified Ann Arbor staging system
Both Hodgkin and Non-Hodgkin lymphoma are staged according to a modified Ann Arbor classification.
This staging system is based on the anatomical extent of disease and is divided into stages I-IV (see table).
Patients with HL are subdivided according to the absence (A) or presence (B) of disease related symptoms (so called B symptoms), because this can influence the choice of therapy.
Bulky disease can be a negative prognostic factor for some lymphomas.
For HL it is defined as a single nodal mass ≥ 10 cm or > 1/3 transthoracic diameter at any level of thoracic vertebrae on CT.
For NHL this definition is not unambiguously, a variety of sizes have been suggested for different subtypes..
Therefore, the recommendation for HL and NHL is to record the longest measurement on CT scan.
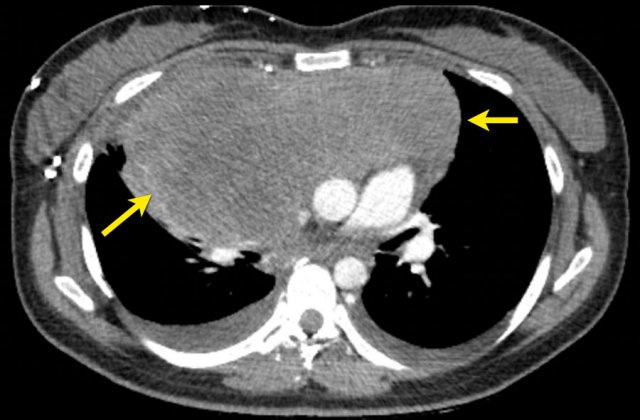
CT image in a 28-year-old female with Hodgkin lymphoma stadium II ‘bulky’.
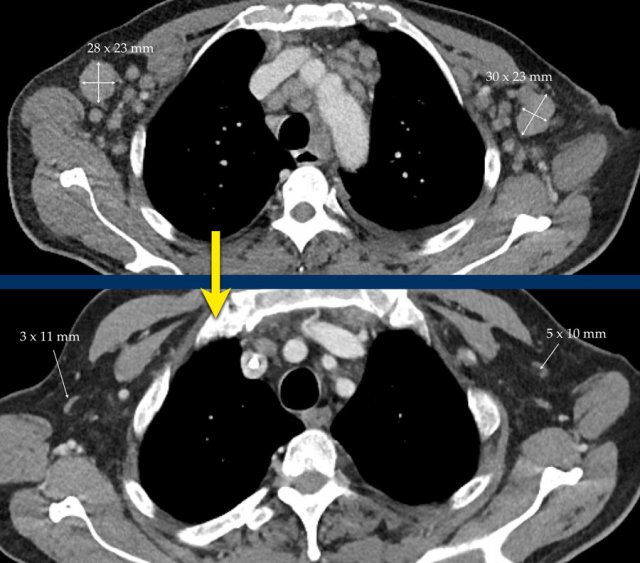
Example bi-dimensional measurement with perpendicular diameters for calculating the sum of the product of perpendicular diameters for multiple lesions.
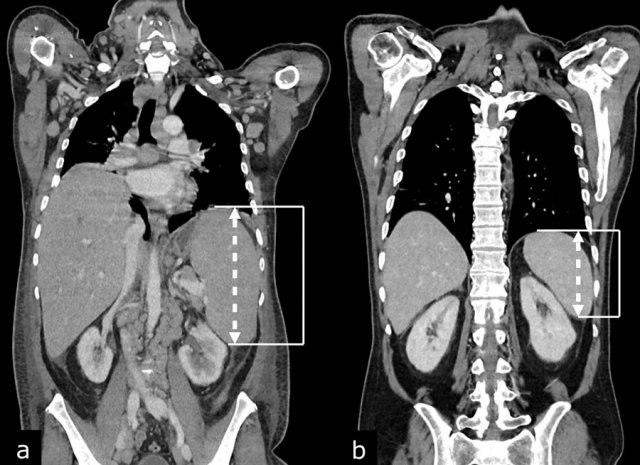
CT images in a 56-year-old male with diffuse large B-cell lymphoma (DLBCL) before (a) and after (b) treatment with R-CHOP chemotherapy.
Coronal reconstructed CT images in the same patient show hepatosplenomegaly before (a) and normalization of hepatic and splenic size after (b) treatment with R-CHOP chemotherapy. Splenomegaly is defined as a vertical length >13 cm.
Hepatic size is not a reliable measure of hepatic involvement by lymphoma.
Response assessment
For response assessment the SPD after treatment is compared to the SPD at baseline.
Four response assessment categories are determined; complete remission (CR), partial response (PR), stable disease (SD) and progressive disease (PD) (table).
To achieve CR no findings of lymphoma should be present, and all target nodes should have a LDi ≤ 1.5 cm. For PR the SPD of up to 6 target lesions should decrease ≥ 50%, there should be no increase in non-measured lesions and splenic size should be regressed > 50% in length beyond normal.
SD is defined as < 50% decrease from baseline SPD of up to 6 target lesions.
To meet the criteria for PD just a single target lesion should increase ≥ 50% in product of perpendicular diameters (PPD) from nadir (smallest PPD during treatment), splenomegaly progresses or occurs, clear progression of pre-existing non measurable lesions is determined or new lesions occur.
- LDi: longest diameter
- SPD: sum of the product of perpendicular diameters for multiple lesions
- PPD: cross product of the LDi and perpendicular diameter
- SDi: shortest axis perpendicular to the LDi
Splitting and confluencing lesions
When a lesion splits during follow-up examination, the individual product of the perpendicular diameters (PPDs) of the nodes should be summed together to represent the PPD of the split lesion.
Likewise, if lesions become confluent, the PPD of the confluent mass should be compared with the sum of the PPDs of the individual nodes, with more than 50% increase in the PPD of the confluent mass compared with the sum of individual nodes necessary to indicate progressive disease.