Endometrial Cancer - MR staging
Stephanie Nougaret¹, Doenja Lambregts², Annemarie Bruining² and Margriet de Haan²
Dept. of Radiology, ¹Montpellier Cancer Centre, France and ²the Netherlands Cancer Institute
Publicationdate
In this article we describe the role of MRI for the
local staging of endometrial cancer.
In addition to clinical and pathological examination, MRI has an important role in identifying patients with
advanced disease and thereby to guide surgical treatment planning.
MRI also
plays a role to select patients eligible for fertility-preserving strategies.
We will discuss:
- MR reporting checklist for endometrial cancer staging
- Interpretation and reporting pitfalls.
- MR anatomy relevant for endometrial cancer staging and treatment planning.
- MR protocol including the role of functional imaging
sequences like DCE and DWI.
- Overview
of current FIGO staging.
Introduction
Endometrial cancer represents the fifth most common cancer type in women and - together with cervical cancer - accounts for 0,7% of all cancer related deaths [ref].
The primary
treatment for endometrial cancer is surgical resection.
The key risk factors in endometrial cancer to
assess on imaging are the depth of
myometrial invasion, invasion into the cervix, bladder or rectum and lymph node
involvement.
Clinical
factors include obesity, tamoxifen use, hyperinsulinemia, prolonged exposure to
unopposed estrogen often caused by nulliparity, and infertility associated with polycystic
ovarian syndrome.
Although most cases of endometrial cancer are sporadic, 5-10% are hereditary,
usually related to the Lynch syndrome.
Endometrial cancers are mostly endometroid adenocarcinomas (80-90%), which have a relatively good prognosis. The remaining 10-20% of endometrial cancers are mainly serous carcinoma, clear cell or carcinosarcoma which all have a poorer prognosis.
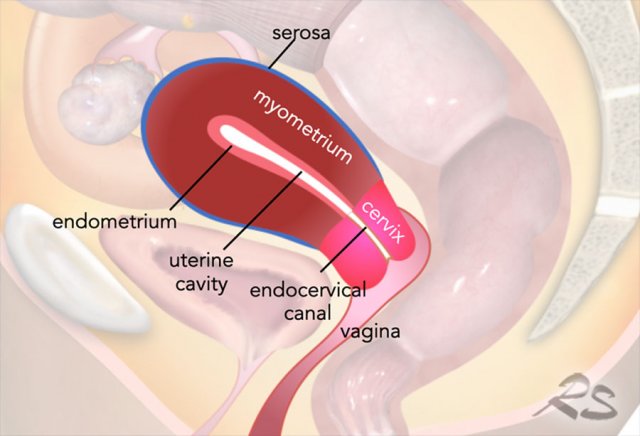
MR Anatomy
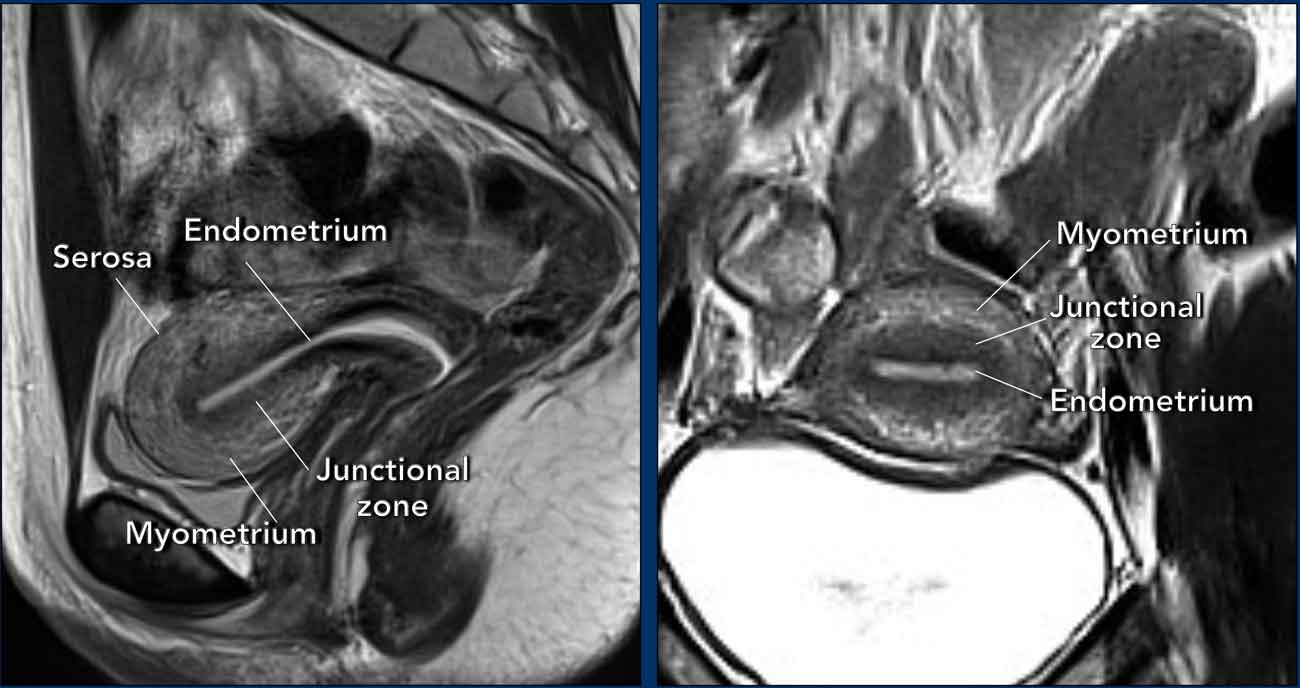
Uterine anatomy is best displayed on T2-weighted MRI.
During the
reproductive period, three distinct zonal layers can be recognized: the
endometrium (high signal), the junctional zone (low signal), and the myometrium
(relatively high signal).
The outer surface of the uterine myometrium, the
serosa, can be recognized as a thin hypointense line.
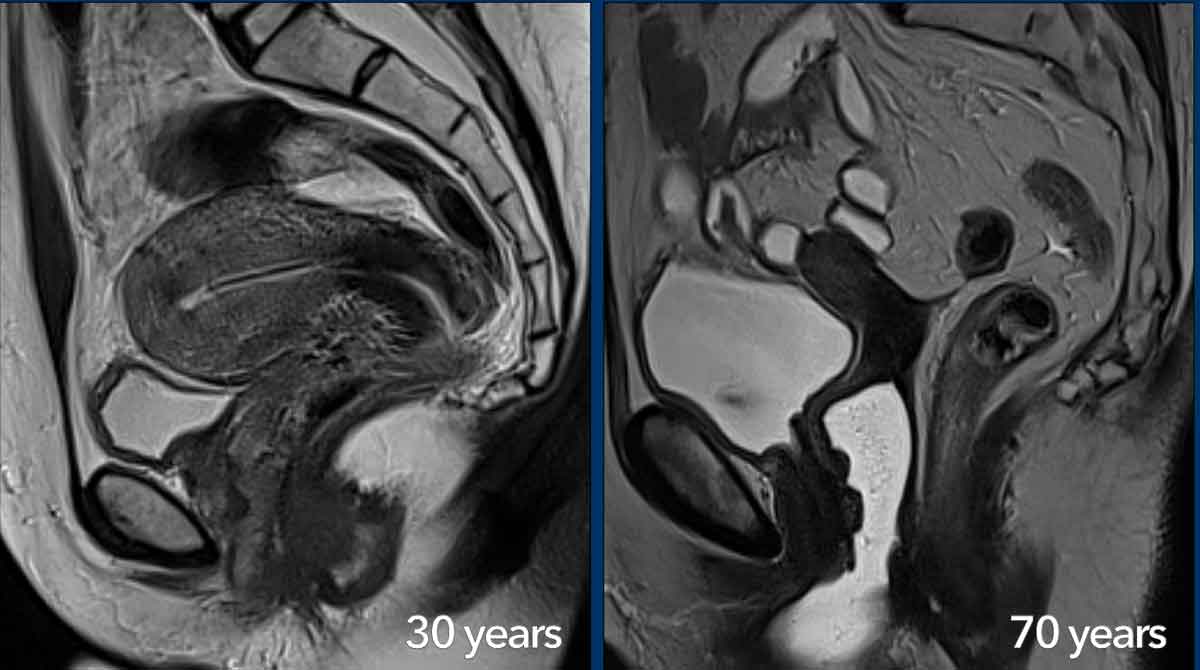
The zonal MRI anatomy of the uterus and cervix varies with age.
During the reproductive age the different layers of the uterus and cervix are well
recognizable and the muscular part of the uterine wall can be highly
vascularized.
In this 30-year-old woman an IUD (recognized as a hypointense linear structure)
is present in the uterine cavity.
In
postmenopausal women the zonal anatomy becomes less visible and the cervical
stroma, junctional zone and myometrium appear more homogeneously hypointense on
T2W-images.
With age, the female reproductive organs gradually become smaller with
a more pronounced loss in volume for the uterus compared to the cervix.
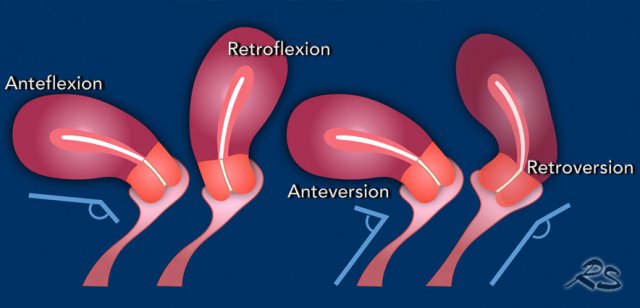
The position of the uterus can vary depending on the angle between the cervix and uterus, i.e. anteflexion or retroflexion and the angle between the vagina and cervix, i.e. anteversion or retroversion.
Staging Endometrial Cancer
MR reporting checklist
The MRI report in endometrial cancer should address the key risk factors listed in the table in order to determine the most appropriate treatment strategy.
Additional factors that are relevant for surgical planning and that should be included in the report are:
- Total uterine size
- Benign conditions such as endometriosis or leiomyoma
- Anatomical variants
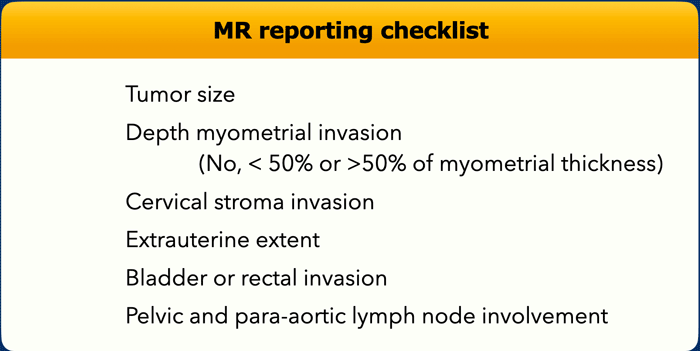
Tumor size
Depending on the age of the patient, endometrial
tumors can present mildly hyperintense (see image) or hypointense (in younger
patients) compared to the normal myometrium.
They are typically well-recognizable
on T2 weighted images.
The morphology of the tumor can vary from a well-delineated
mass to a more diffuse tumoral thickening growing along the endometrial
lining.
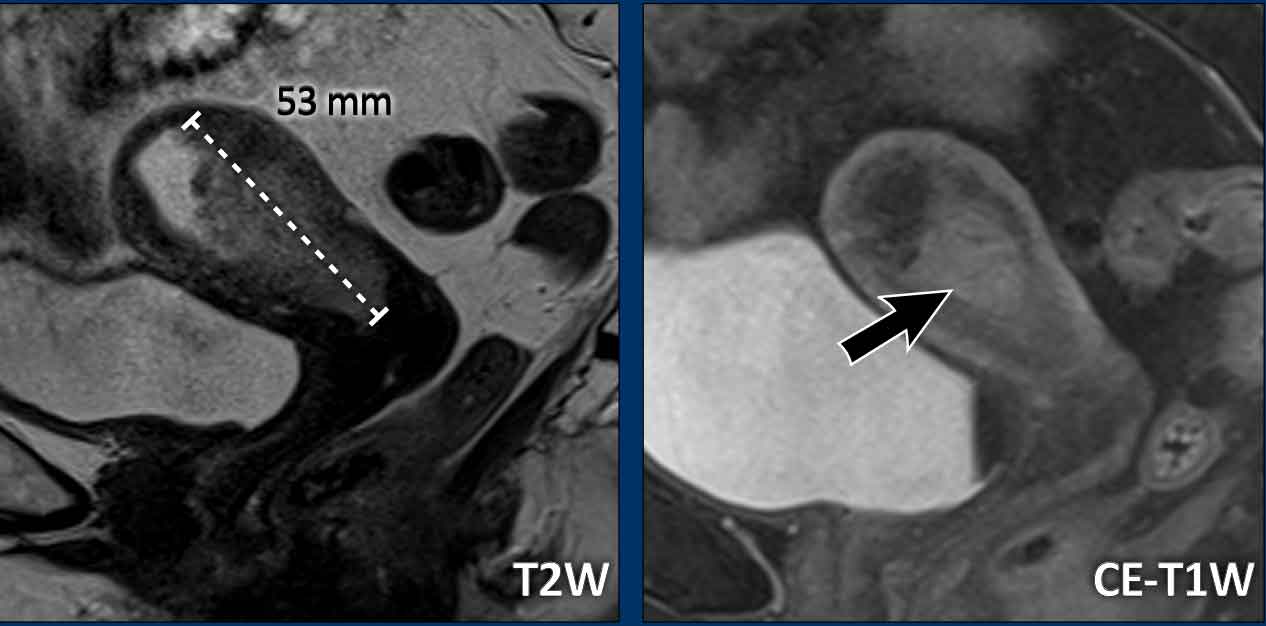
Image
Example of a mass-forming endometrial tumor, which
are typically easiest to measure. It is important to measure the longest tumor
diameter.
In this case, the longest tumor diameter is best visualized on the
sagittal plane.
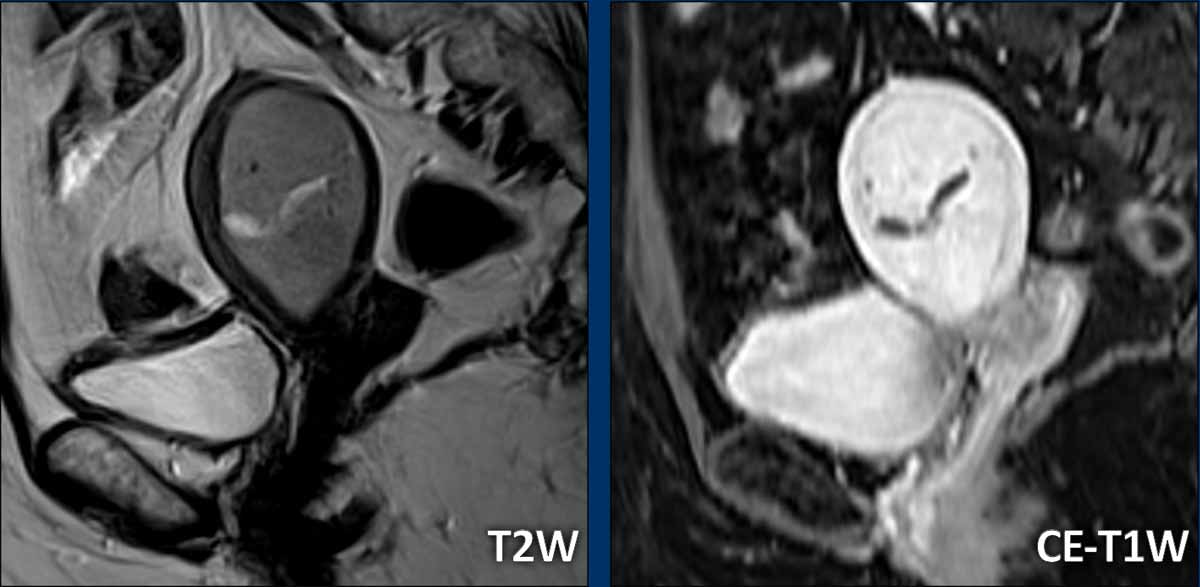
In cases with more diffuse tumoral thickening along the endometrial
lining, it can be more difficult to measure the tumor size.
Check the tumor in
multiple planes and look for the longest possible tumor size.
In this case, the
longest tumor diameter is best visualized in the axial plane (figure).
Note that the normal endometrial thickness
varies between pre- and postmenopausal women, with various cut offs reported in
literature:
- Premenopausal: ≤16 mm (varying thickness during different phases of menstrual cycles)
- Postmenopausal (usually 1-5 mm):
- No vaginal blood loss: < 11 mm
- Vaginal blood loss and/or on
tamoxifen: < 5 mm
These thresholds have been proposed to warrant
further gynaecological evaluation.
Final decisions
about further investigations should always be made on a case-by-case basis,
taking into account clinical symptoms, tamoxifen use and risk factors for
developing endometrial pathology.
Sometimes tumors are less well visible and
difficult to delineate on T2-weighted images.
In such cases the DWI (and ADC
map) and T1 post contrast series can help to better delineate and measure the tumor.
Most endometrial cancers show distinctly high signal on high b-value DWI and are hypovascular compared to the surrounding myometrium on contrast-enhanced T1-weighted images.
Atypical enhancement patterns
Note that not all endometrial tumors are hypovascular compared to the myometrium.
The case on the left shows an example of an endometrial tumor with sarcomatoid components which can show strongly enhancing components.
Another example of a tumor that does not
show a typical hypovascular appearance, but instead shows diffuse enhancement that is almost similar to the enhancement of the surrounding
myometrium.
This is a case of high grade endometrial stroma sarcoma.
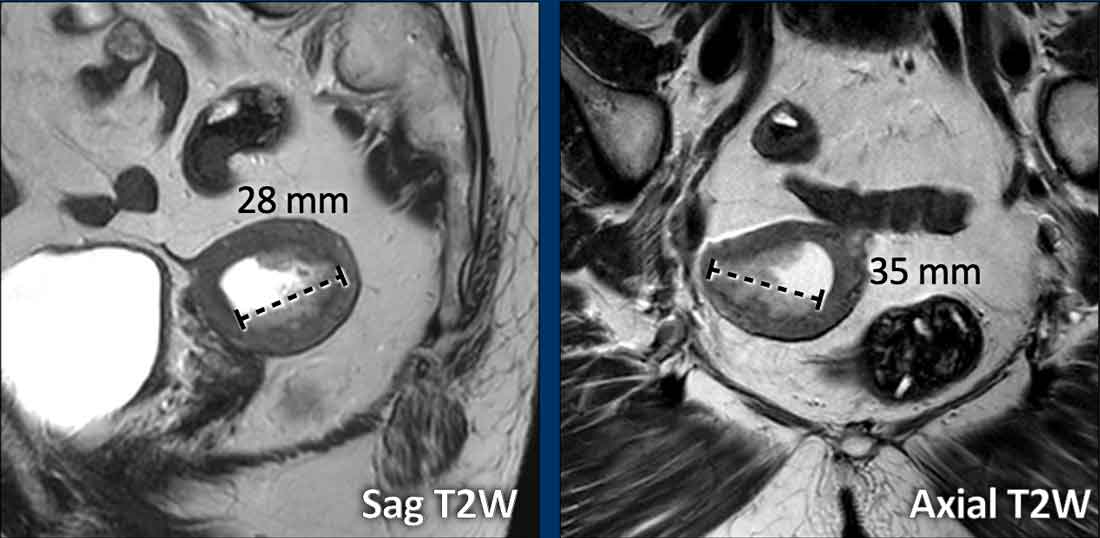
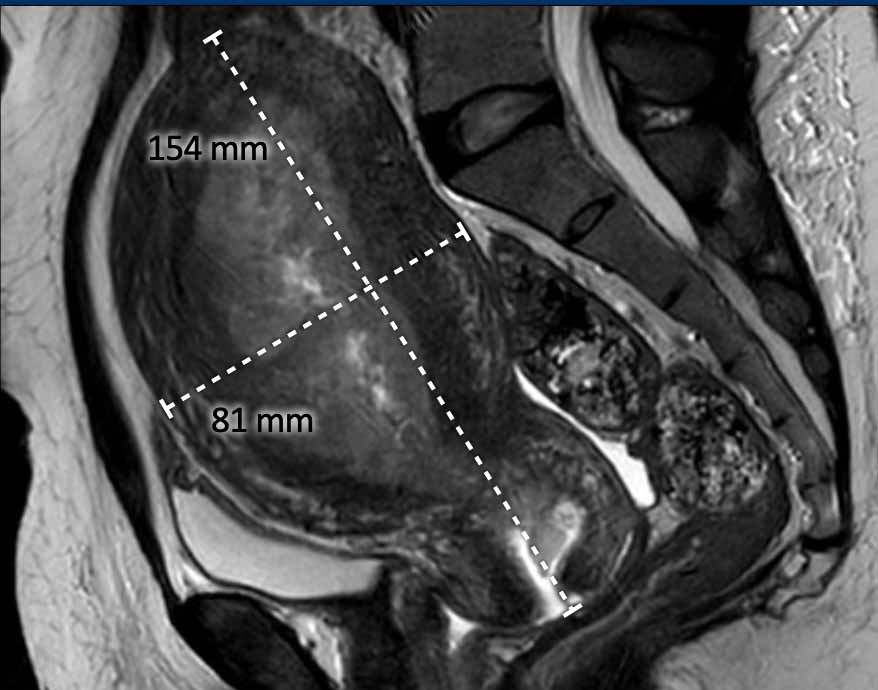
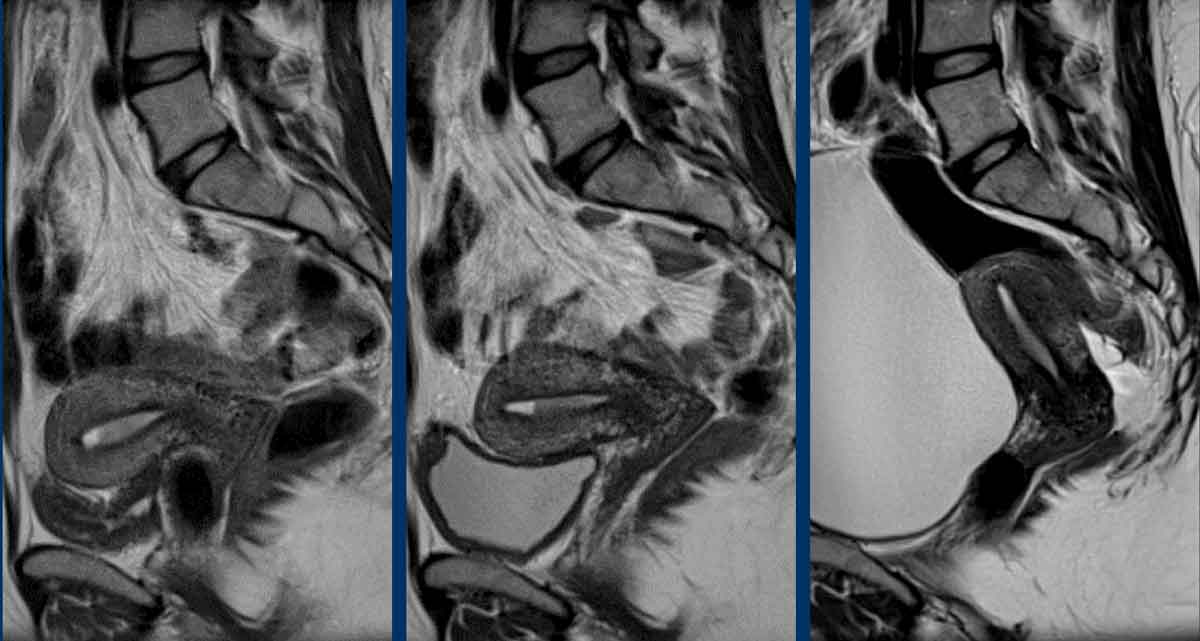
Total uterine size
The
total uterine size is
important to report as this will impact the surgical strategy of a transvaginal
versus laparoscopic or open transabdominal approach.
If the total uterine size is too large (e.g. >10 cm), this will be a contra-indication to perform transvaginal or laparoscopic
surgery.
The uterine size is typically measured in the sagittal plane in 2 dimensions: craniocaudal (including the cervix) x anteroposterior (figure).
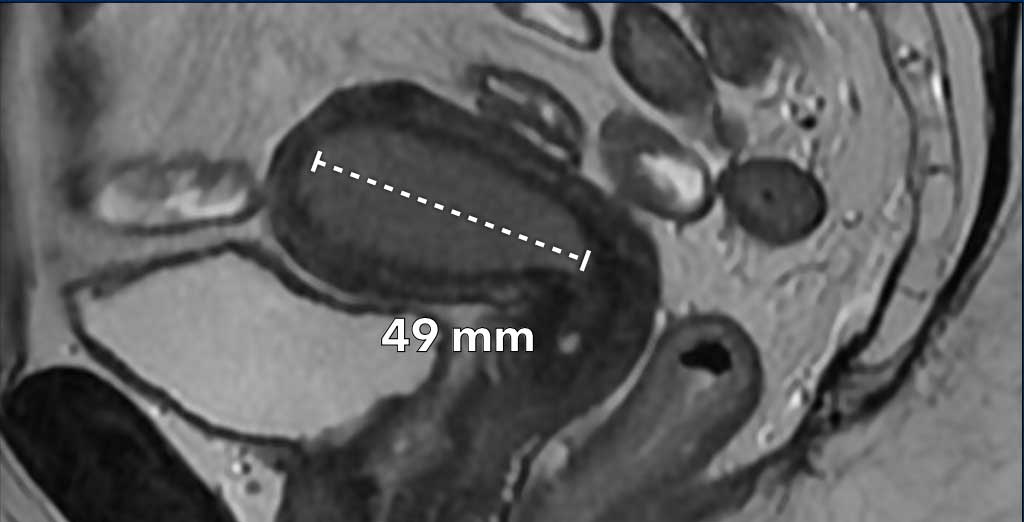
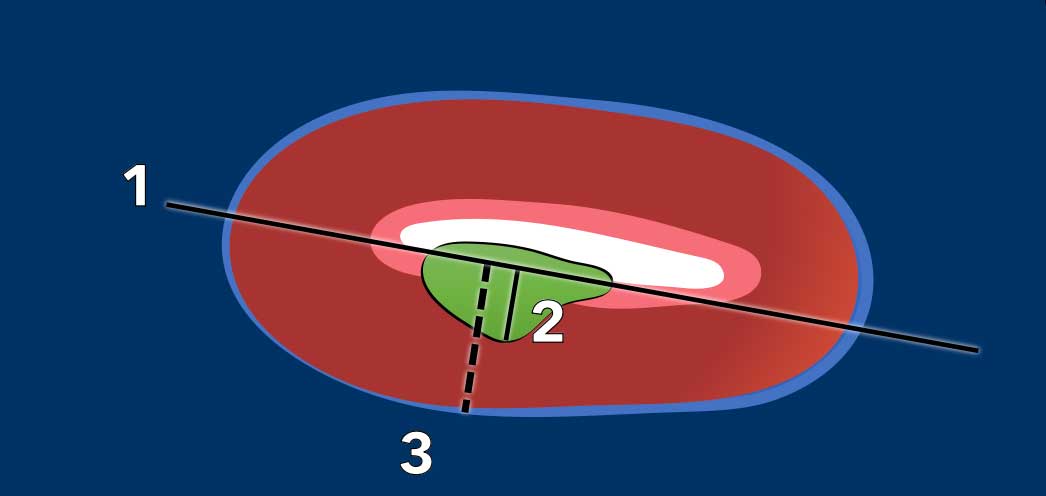
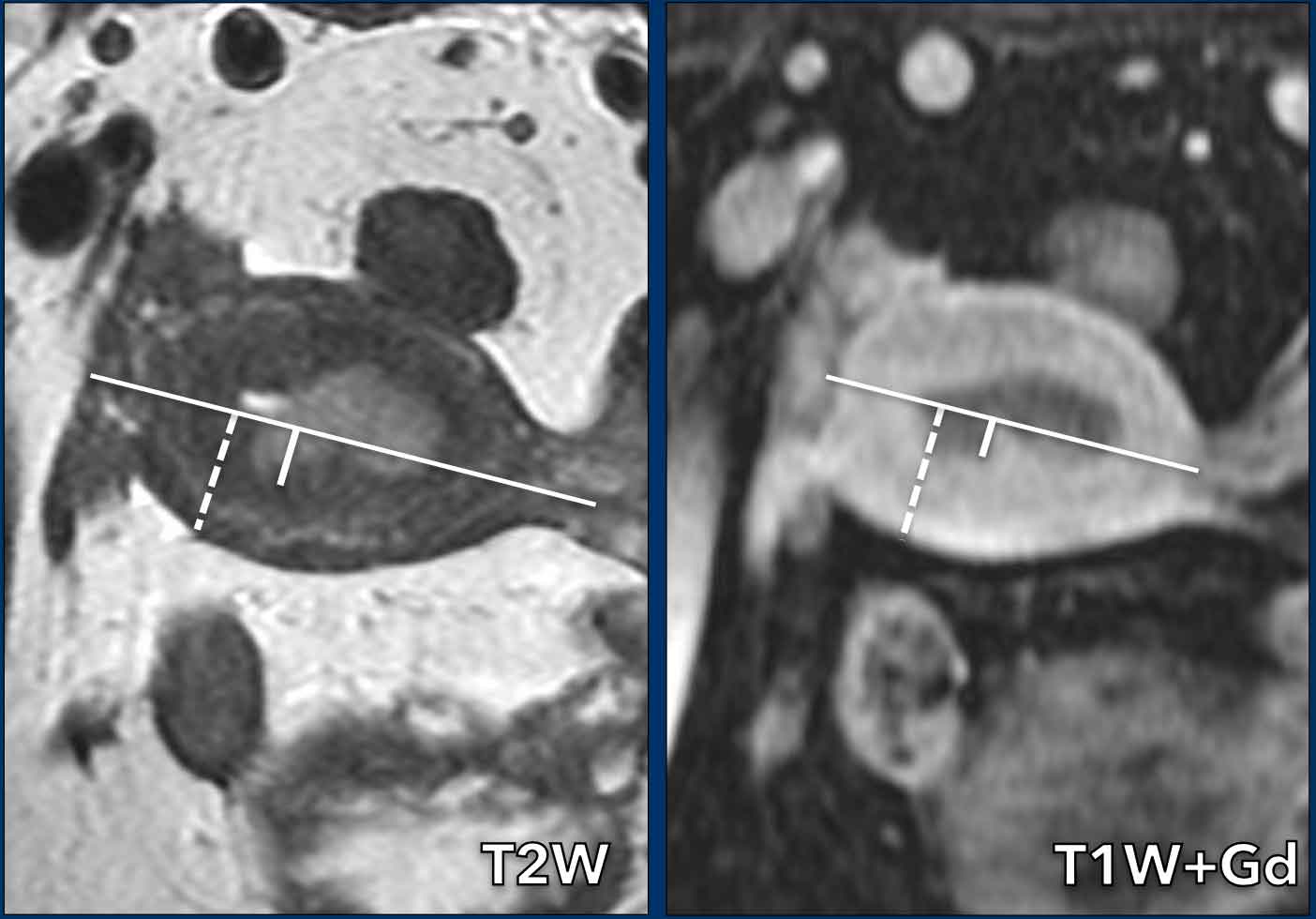
Myometrial invasion
Measuring the depth of myometrial invasion is typically done using a combination of the sagittal and the perpendicular axial plane.
It entails a 3-step approach (figure):
- draw a line parallel to the inner myometrium
- measure the maximum tumor extent into the myometrium
- determine the full myometrial thickness (dashed line)
The ratio between 2 and 3 represents the percentage of myometrial invasion.
An invasion depth of < 50% of the full myometrial thickness is considered ‘superficial’ invasion.
An invasion depth of > 50% is considered deep invasion which is associated with a higher risk for lymph-vascular space invasion, which in turn is associated with higher tumor grade, risk for nodal metastases and increase risk for tumor recurrence.
Note that according to the new FIGO classification
it is important to explicitly mention the presence or
absence of any myometrial invasion, whether superficial or deep.
The absence
of myometrial invasion is particularly relevant to select patients eligible to
undergo fertility sparing treatment (see section on
fertility preservation below).
Images
Myometrial invasion is shown as a disruption of the normal low signal of the junctional zone on T2W-image and as interrupted enhancement of the endometrial-myometrial
interface on post-contrast images, where endometrial cancers appear hypointense
compared to the enhancing myometrium.
The myometrium has a full thickness of 21 mm, but the
invasion into the myometrium is only 6mm (6/21=28%, indicating < 50%
invasion).
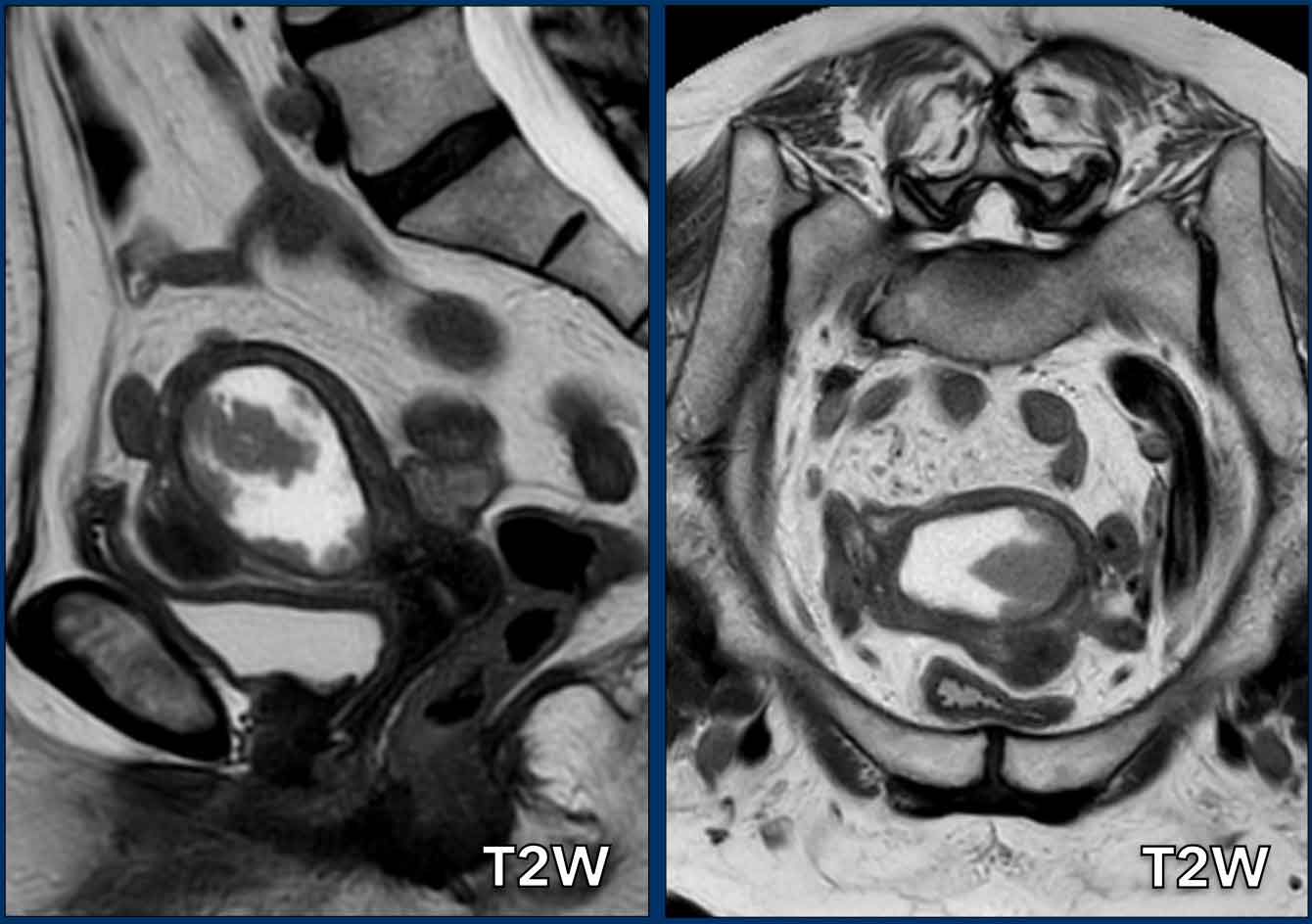
Pitfall - Expansion versus invasion
It is important to differentiate expansion from invasion.
Sometimes there can be enormous expansion without any invasion.
Images
There is obvious expansion of the uterine cavity, but without any signs of
myometrial invasion.
Note how we can nicely recognize a completely intact
hypointense junctional zone to confidently rule of invasion of the myometrium.
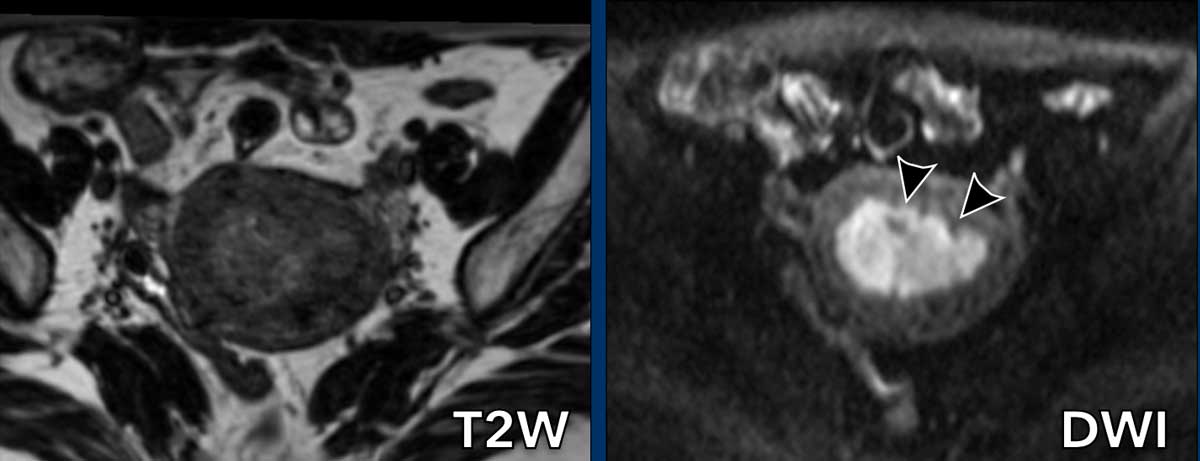
Benefit of DWI
Sometimes the tumor is
difficult to delineate on T2W-images because it is almost isointense compared to
the myometrium.
In such cases, DWI helps in differentiation between tumor and
myometrium.
Images
On the T2w-image the tumor is almost isointense to the myometrium.
The DWI-image shows that there is myometrial invasion but less
than 50%.
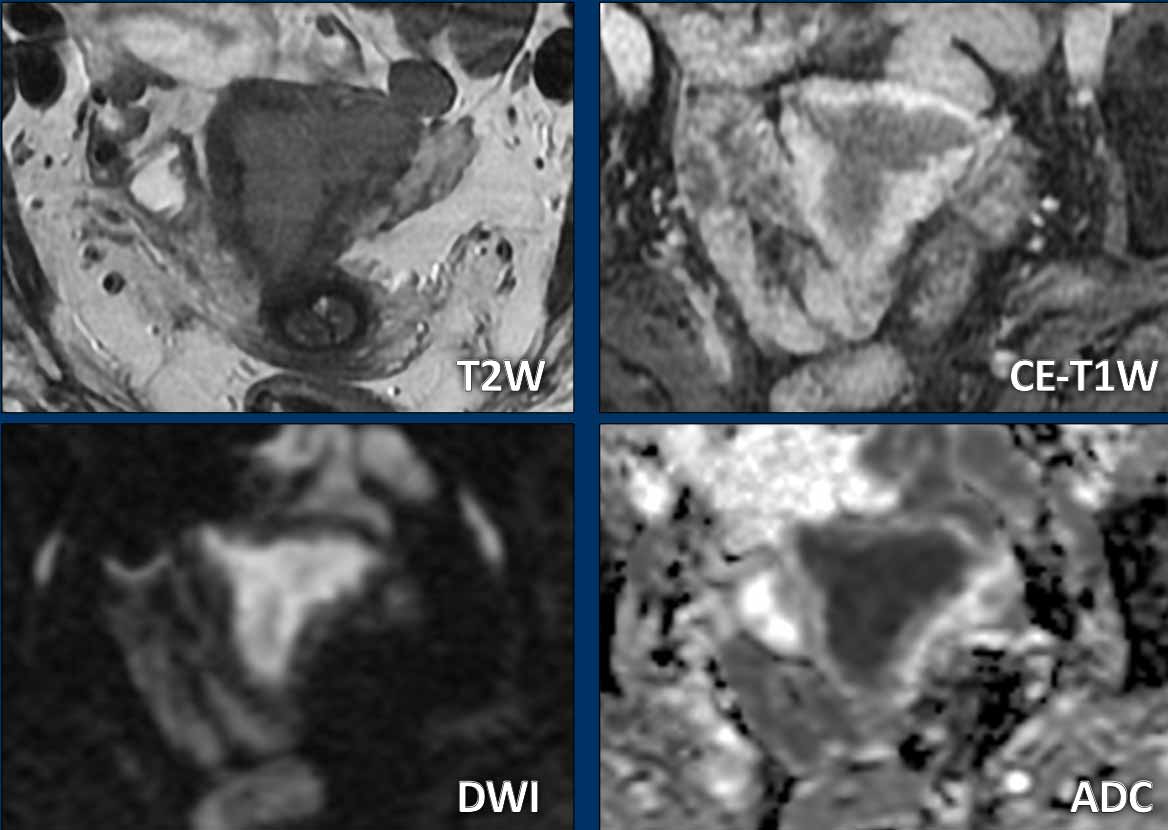
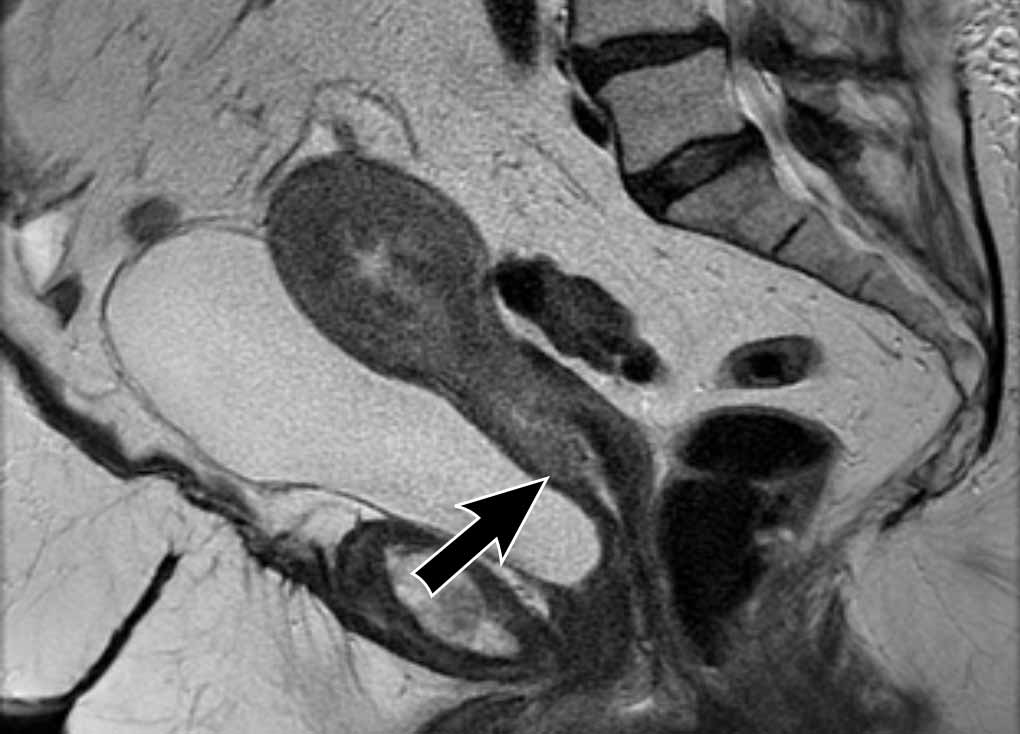
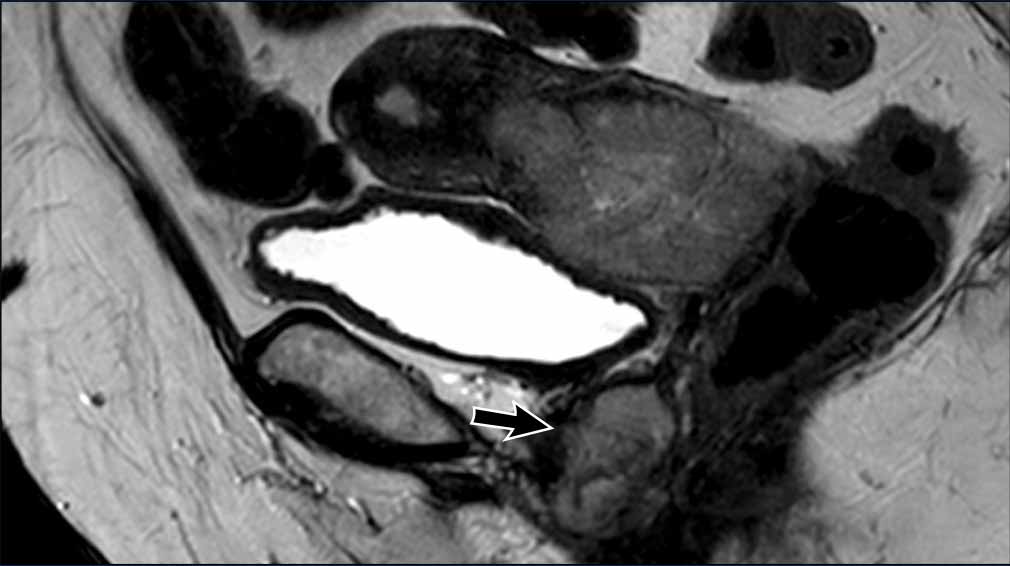
Cervical Stromal invasion
Cervical
stromal invasion can be detected as an extension of tumor signal with
corresponding disruption of the normal low signal of the cervical stroma on T2W-images.
It can also be appreciated as a disruption of the normal cervical stromal
enhancement on CE images, or as extension of high tumor signal on high b-value
DWI.
Image
There is an endometrial tumor that invades the cervical stroma
anteriorly.
There is extension of the intermediate tumor signal that
disrupts the normal hypointense signal of the cervix (arrow).
Pitfall – endocervical tumor protrusion
Cervical stromal invasion should be
distinguished from tumor protrusion into the cervical canal.
Tumor
protrusion can cause widening of the cervical canal with consequent thinning of
the cervical stroma due to mass effect.
Image
Note that there is no actual extension of tumor signal into the cervical
stroma.
Therefore there is no cervical stromal invasion.
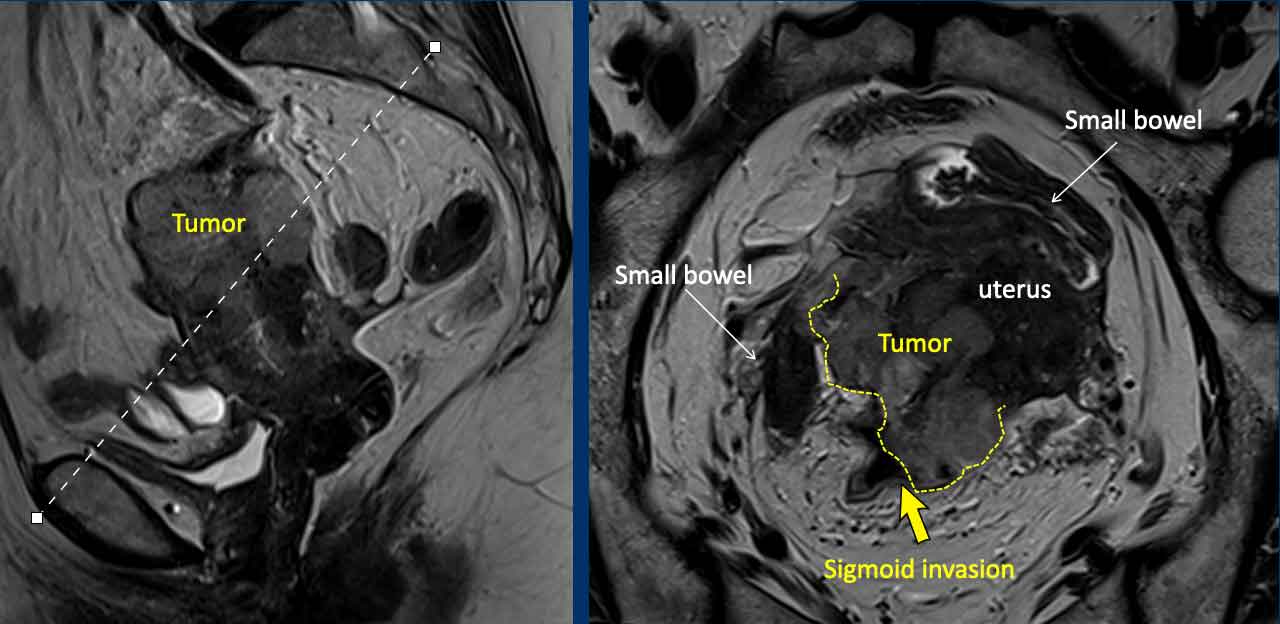
Extrauterine extension (incl. bladder and rectal extension)
The vast
majority of endometrial cancers are confined to the uterus at the time of
diagnosis, as clinical symptoms (blood loss) typically occur at an early stage.
Extrauterine extension and invasion of the bladder or rectal wall are therefore
rarely observed on MRI.
When we do see it, it entails advanced stage disease which should be clearly
stated in the report as it will affect the surgical approach.
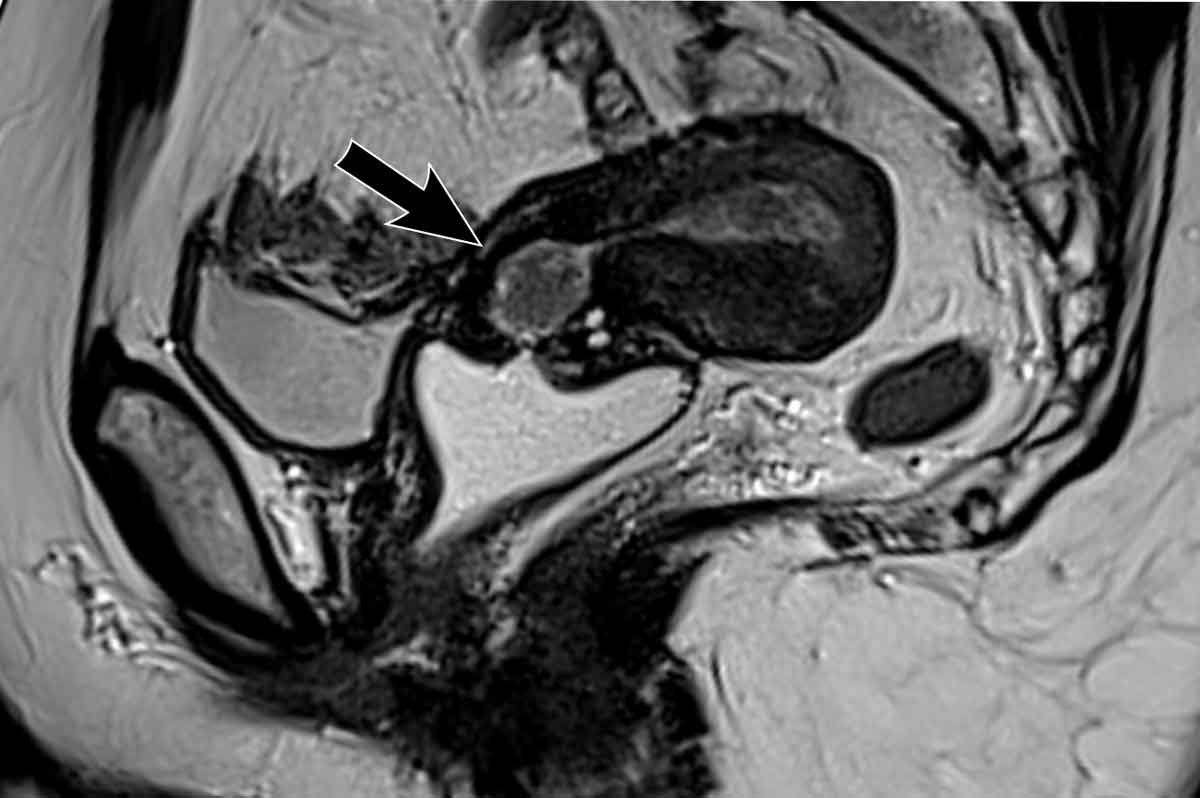
Image
This is a rare example of a locally advanced endometrial cancer that grows beyond the uterine serosa on the dorsal side where it invades the sigmoid colon (arrow)
Another example of an endometrial tumor (with serous components at histology) that shows extensive cervical stroma invasion and a separate tumor deposit (metastasis) in the vaginal wall.
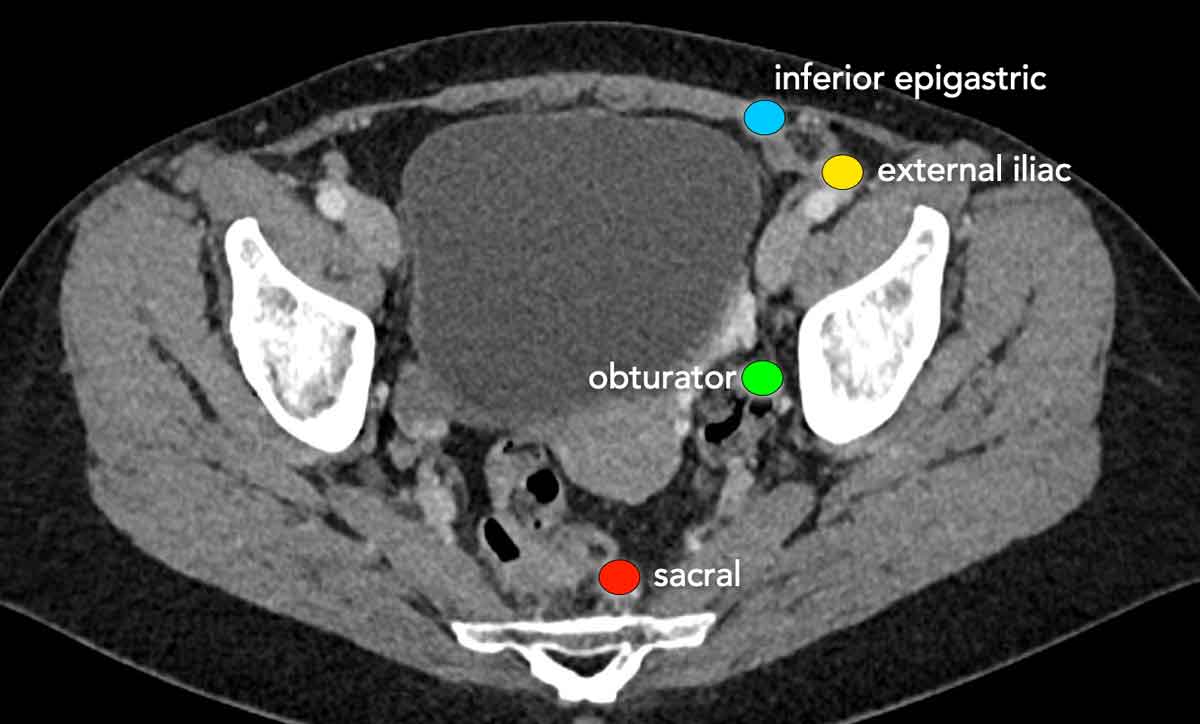
Lymph node staging
When staging endometrial cancer, the regional (N-stage) lymph nodes include all lymph nodes in the pelvis except the inguinal lymph nodes, which are considered distant (M-stage) nodes.
Para-aortic nodes
up to the level of the renal veins are also considered regional nodes.
Nodes
above the level of the renal veins are considered distant metastases.
In addition to MRI, lymph node staging in endometrial cancer is generally performed with the use of sentinel lymph node mapping and/or lymphadenectomy during surgery. PET-CT is not routinely performed despite the fact that it has an excellent diagnostic performance for detecting lymph node metastases preoperatively (reference).
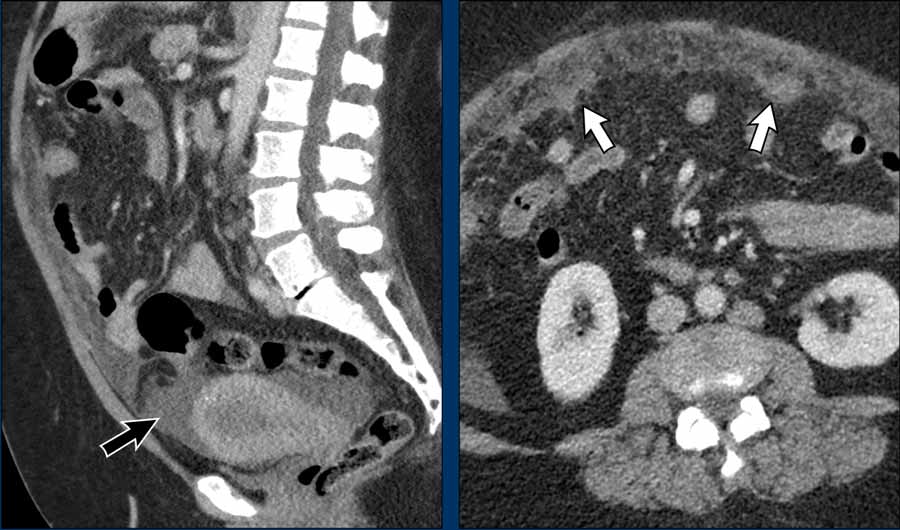
Hematogenous metastases
Endometrial cancer most often metastasizes to the peritoneum or lung. It is not uncommon that the local disease is relatively limited, but there is still hematogenous tumor spread.
Images
Although the endometrial tumor is difficult
to recognize on CT (remember that for local staging we need MRI) it appears to
remain confined to the uterus with no signs of a mass extending beyond the
uterus.
There are, however, obvious signs of peritoneal metastases as there is
diffuse ascites (black arrow) and clearly visible omental cake (white arrows).
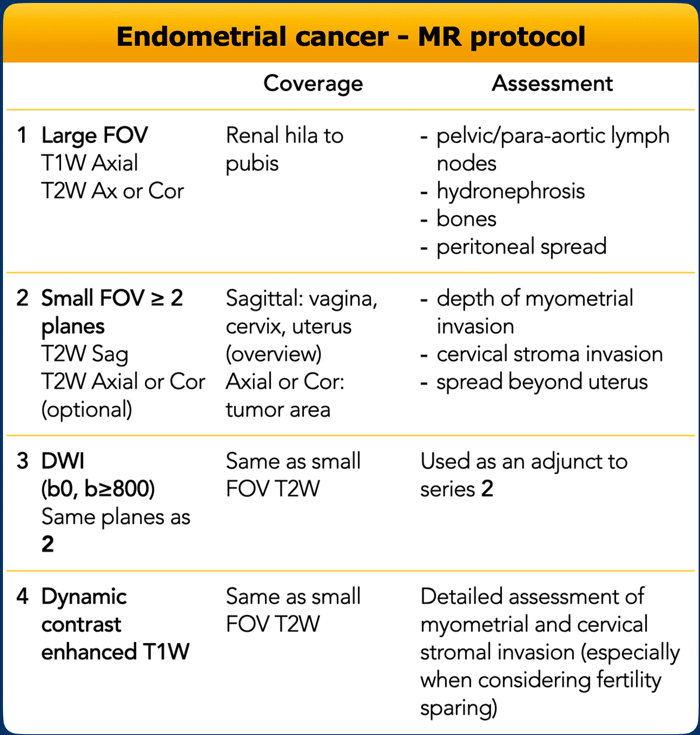
MR protocol
The scanning parameters are shown in the table.
The MR protocol also includes:
- Use a field strength of 1.5T or higher, using a pelvic phased-array coil.
- Patient in supine position
- Scheduling according to the menstrual cycle is not required.
- Fasting (4-6 hours)
- Empty bladder
- Use of anti-peristaltic agents (Buscopan or Glucagon)
- Saturation bands on the subcutaneous fat both anterior and posterior is recommended.
- Contrast-enhanced
images acquired after 2.5 minutes provide the
best contrast between the tumor and the myometrium.
According to the ESUR guidelines the contrast-enhanced images can either be acquired as part of a DCE acquisition or using a single-phase axial acquisition, though with the update of the FIGO guidelines future recommendation may advocate for the routine use of DCE.
DCE-MRI offers the advantage of multiphase imaging:
- Early phase images (30-60 seconds after injection) are optimal to evaluate sub-endometrial enhancement, which is important to assess patients’ eligibility for fertility sparing treatment (see section on fertility preservation below).
- Equilibrium phase images (120-180 seconds) are the best to evaluate the depth of myometrial invasion
- Delayed phase images (4-5 minutes) are optimal for the detection of cervical stromal invasion
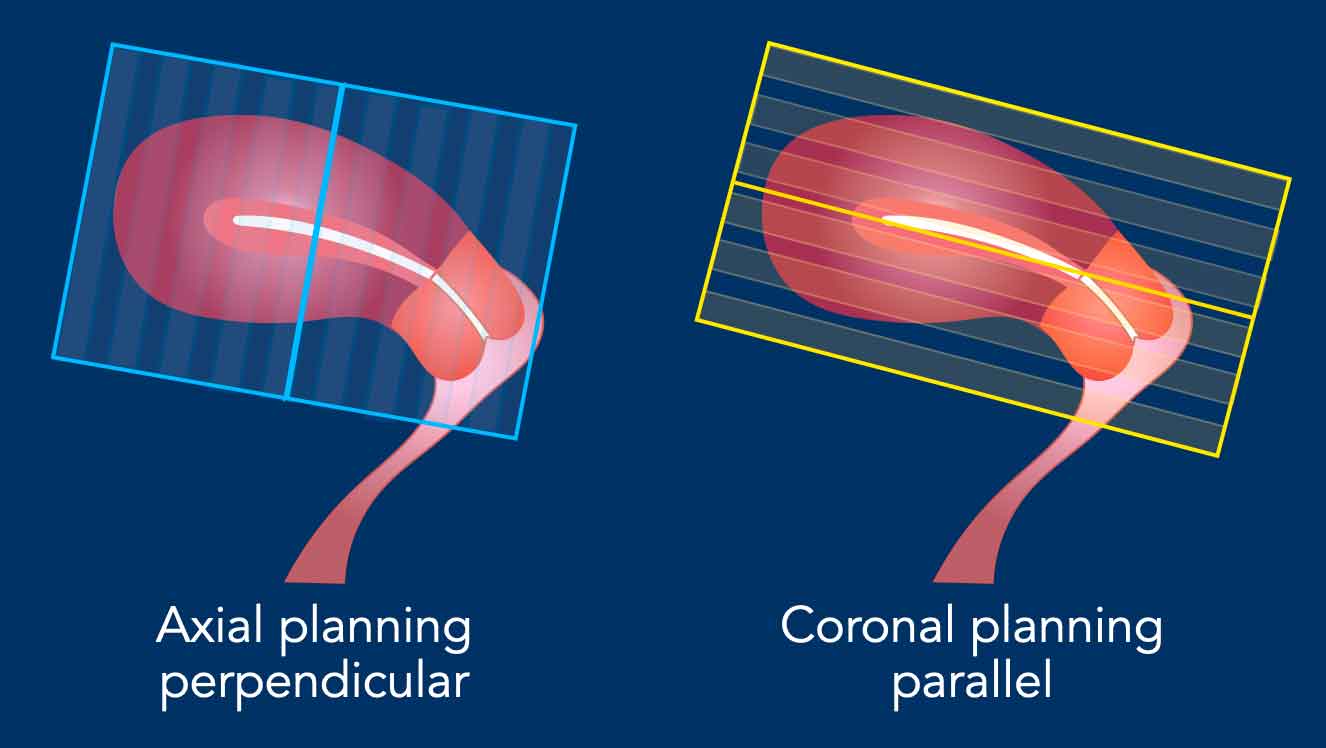
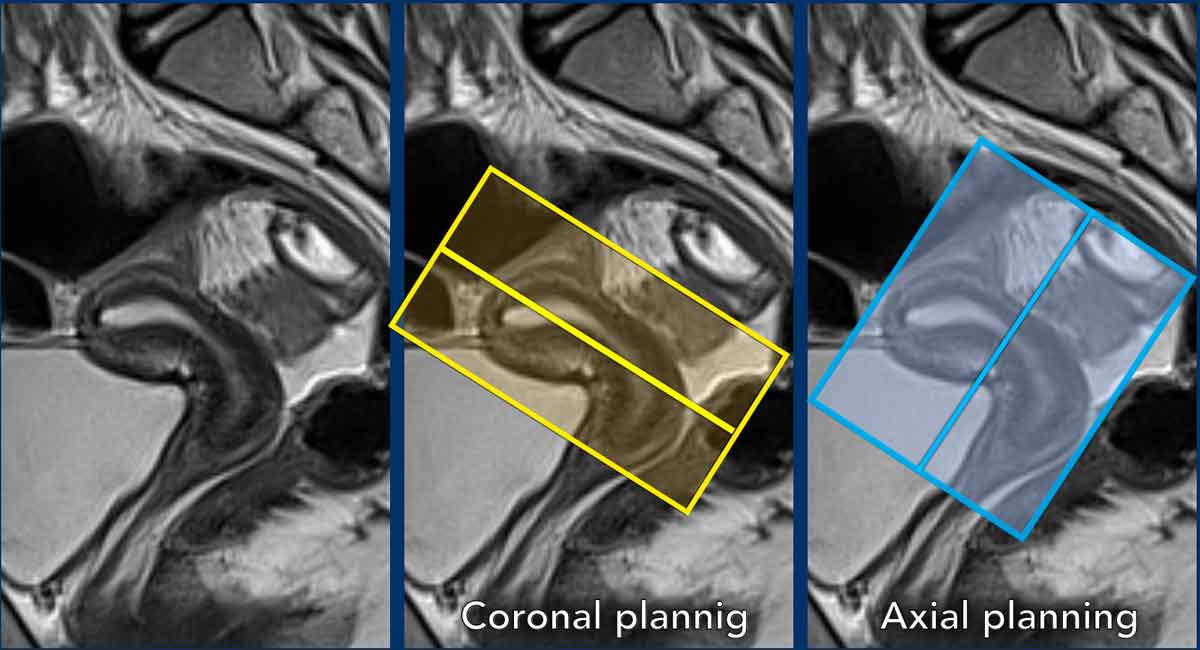
Sequence planning
The MR sequences are planned relative to
the long axis of the uterine cavity.
The axial plane is perpendicular to the long axis of the uterine cavity.
The coronal plane is parallel to the long axis.
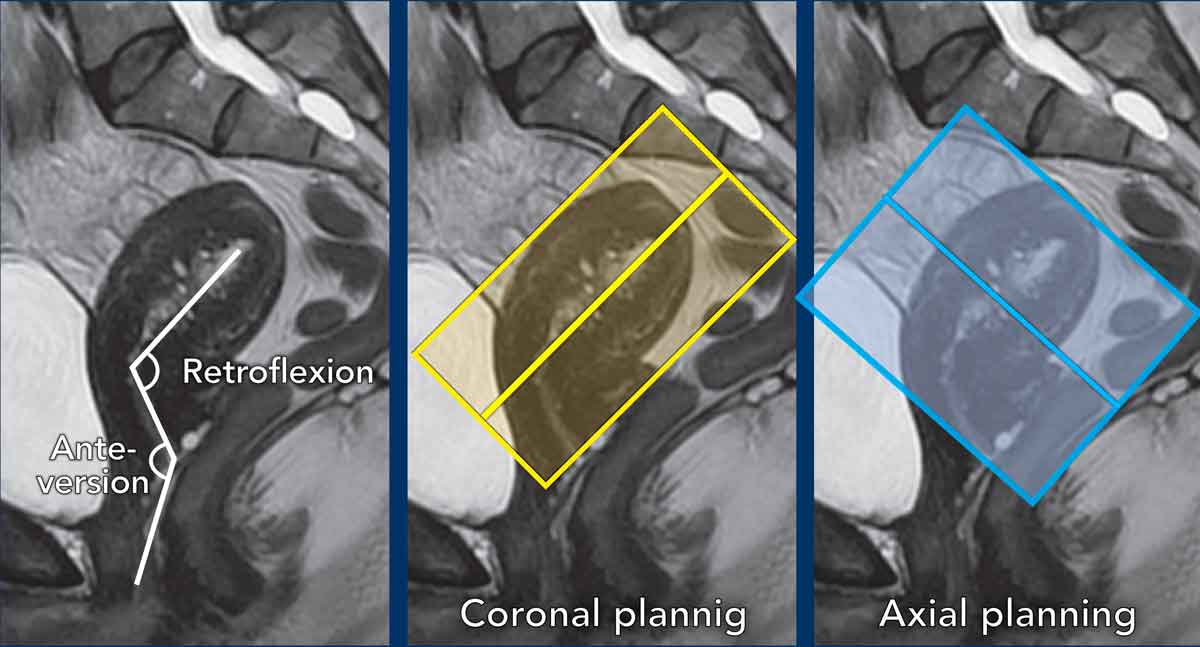
Pitfall: variations in uterine anatomy
The
position of the uterus needs to be taken into account and the perpendicular and parallel MRI
sequences need to be planned accordingly.
In this case there is anteversion of the cervix and retroflexion of
the uterus.
The coronal series are planned parallel to the uterine cavity (yellow box), while the axial series are planned perpendicular to it (blue box).
Here another
example showing the cervix in retroversion and uterus in anteflexion.
See how
this variation in position impacts corresponding sequence planning.
Pitfall: variations in uterine position
Note
that the position of the uterus can vary significantly between and even during
MRI examinations based on for example the degree of bladder filling.
These
variations, as well as variations in version and flexion described above can
pose a real challenge for MRI technicians.
MRI technicians should therefore
receive proper training on how to recognize and handle these variations.
Moreover, when technicians are in doubt, radiologists should be available
during scanning to supervise the examination and offer advice on for example
bladder voiding.
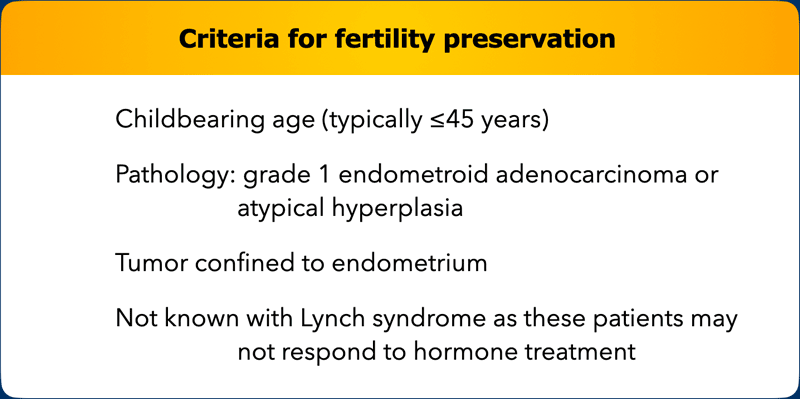
Fertility preservation
For endometrial
cancer, fertility preserving treatment consists of hormonal therapy which is
not considered standard of care treatment.
It is only offered in selected
patients and secondary surgery is typically performed once the family is
complete.
The Table shows the
main selection criteria for fertility preservation in endometrial
cancer.
Confinement of the tumor to the endometrium is confirmed by the
presence of an intact sub-endometrial line on early-phase DCE images (35-40
sec).
As such, DCE
imaging is mandatory to stage endometrial cancer in young patients in whom
fertility preserving treatment is considered as a potential treatment option.
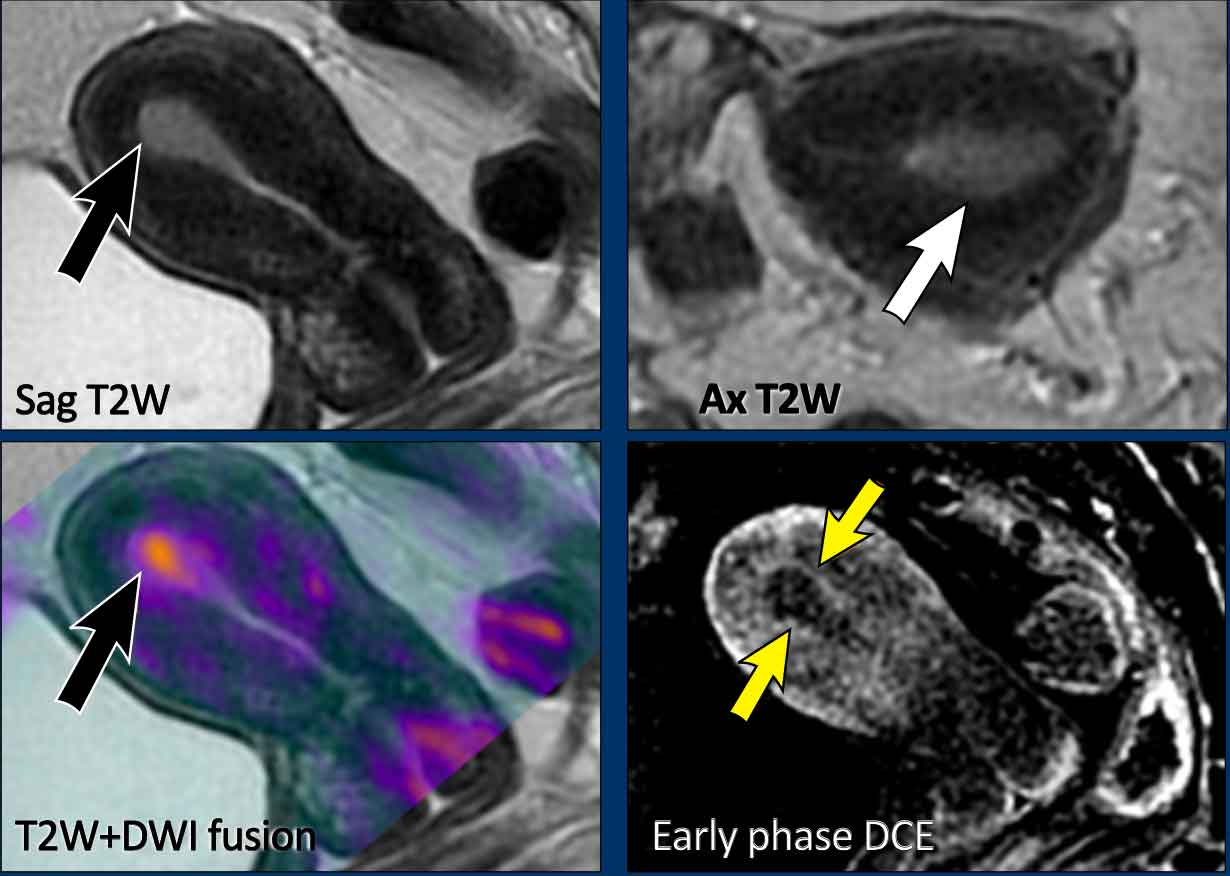
Image
Example of an
endometrial tumor that is confined to the endometrium.
There is a smooth interface between the tumor and junctional zone on T2W MRI.
Though
not routinely done, it can be helpful to fuse the T2W and high b-value
diffusion-weighted images.
In this case the fusion images with the DWI in
color overlay confirm the absence of myometrial invasion.
The most accurate
technique to confirm this is DCE.
Early phase images at 30-60 seconds after
injection are optimal to evaluate sub-endometrial enhancement, which is
important to assess patients’ eligibility
for fertility sparing treatment.
In this case the subendometrial line is intact on the early phase DCE images (arrows), indicating no myometrial invasion.
FIGO stage
The International
Federation of Gynaecology and Obstretrics (FIGO) staging system that is most
commonly used to stage cervical and endometrial cancers was traditionally
designed as a clinical surgical staging system.
However, current evidence and
clinical guidelines recommend to include imaging findings, in particular MRI for staging and treatment planning as it provides crucial information on tumor
size and depth, extent of invasion into surrounding organs and structures, and
lymph node status, which are essential in choosing the most appropriate
treatment strategy.
An overview of the current 2023 FIGO stages for cervical
and endometrial cancer is provided in the overview Table on the left but we
refer readers to the complete FIGO guidelines for more detailed info [ref].
Clinical classification systems (such as
FIGO) are constantly evolving to accommodate new clinical insights, novel
prognostic markers and treatment developments. For example, the risk
classification and clinical guidelines for endometrial cancer have recently
been updated to take into account different molecular subtypes that can
influence adjuvant treatment recommendations, especially in
high-grade/high-stage tumors.
The new
FIGO 2023 endometrial classification recommends including molecular profiles,
which are divided into four groups (POLEmut; MMRd; p53abn; and NSMP) each with
different prognostic profiles that in the current version of the FIGO staging
system modify the final stage [ref].
Charity
All the profits of the Radiology Assistant go to Medical Action Myanmar which is run by Dr. Nini Tun and Dr. Frank Smithuis sr, who is a professor at Oxford university and happens to be the brother of Robin Smithuis.
Click here to watch the video of Medical Action Myanmar and if you like the Radiology Assistant, please support Medical Action Myanmar with a small gift.