Staging and Treatment of Breast Cancer
Robin Smithuis, Janneke de Bes and Anneke Zeillemaker
Department of Radiology and Surgery of the Alrijne hospital, Leiderdorp in the Netherlands
Publicationdate
Imaging plays a major role in the detection and staging of breast cancer and monitoring treatment.
In this era of rapidly changing management of breast cancer, it is important to be familiar with the staging classification and be aware of the available surgical, radiotherapeutic and oncological management options in order to provide relevant information and so optimize patient care
This article is an overview of breast cancer staging and treatment.
Keep in mind there are local differences in treatment strategies, and that new targeted therapies are currently being developed.
Overview
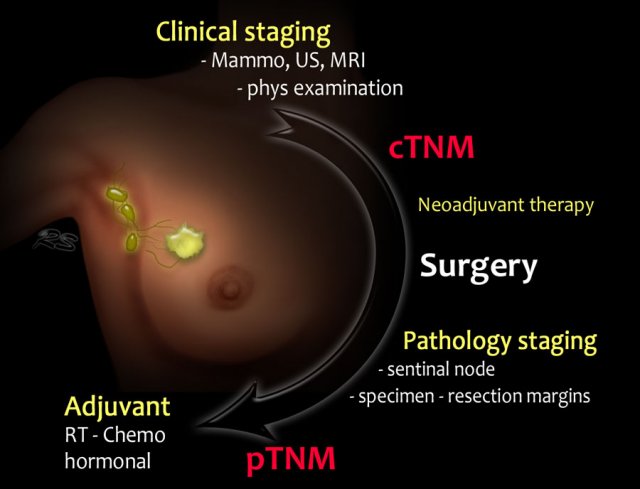
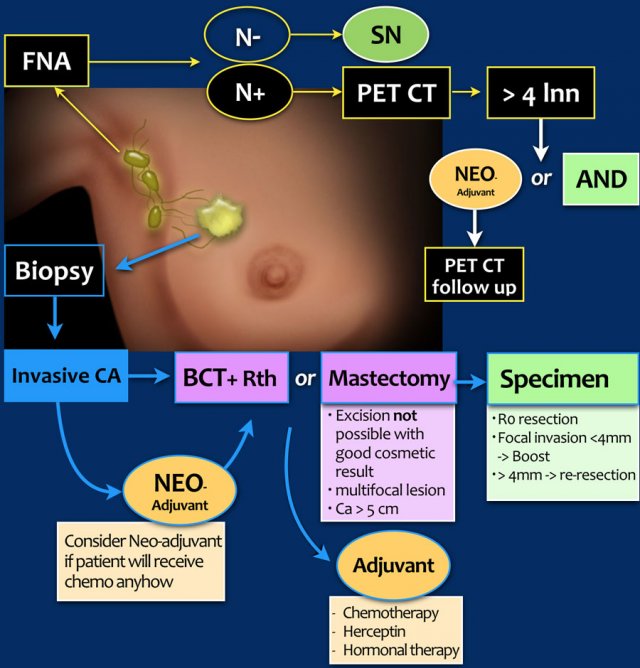
Staging and treatment overview 1
This is a schematic illustration of the staging and treatment in a patient with breast cancer.
- The patient with breast cancer is first clinically staged, which results in a cTNMstage. Treatment planning is then discussed in a multidisciplinary team.
- In many patients, surgery will be the next step. Increasingly, neoadjuvant chemotherapy is given in order to decrease the tumor burden prior to surgery.
- Following surgery, the surgical specimens (tumor, sentinel node or axillary nodes after dissection) are analyzed by the pathologist.
This results in a pTNM-stage. After neoadjuvant therapy the letter y is added, and it is referred to as the ypTNMstage. - Based on the pTNM- or ypTNM-stage, further treatment with adjuvant chemotherapy, radiotherapy and hormonal or HER-2 targeted therapy is discussed in the multidisciplinary team.
- c - clinical
- p - pathology
- y - post neoadjuvant therapy
Every image can be clicked to enlarge.
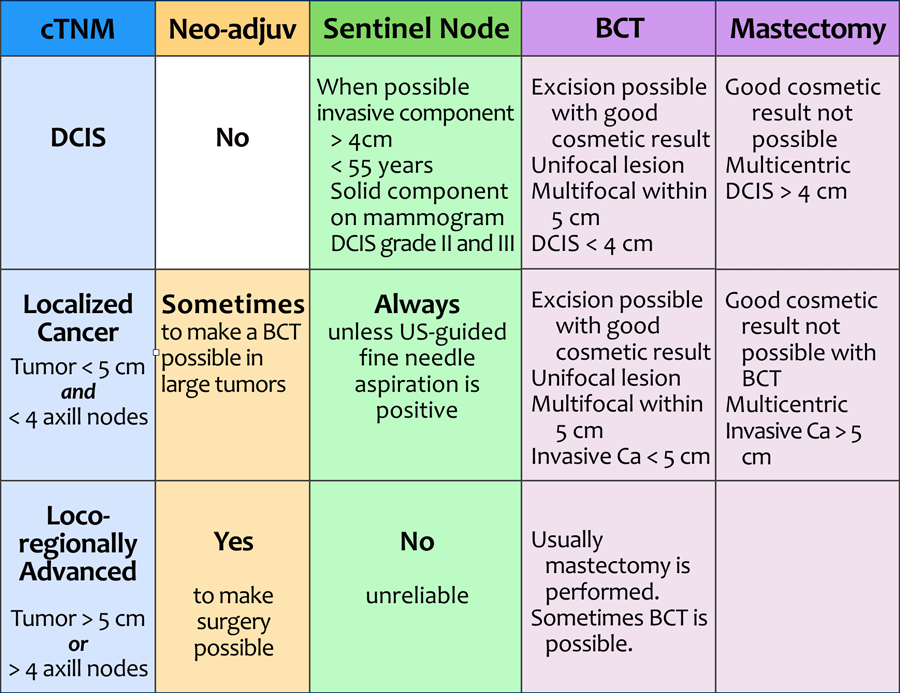
Staging and treatment overview 2
This table demonstrates the staging and treatment of breastcancer in a more schematic way.
cTNM
Breast cancer is first clinically staged.
The cTNM describes the local involvement of the breast (T), regional lymph nodes (N) and systemic disease, i.e. distant metastases (M). Systemic disease (or stage 4 disease) is not included in the table.
This results in the possible diagnoses of DCIS, localized cancer, locoregionally advanced cancer and cancer with distant metastases.
The term 'clinical' in clinical TNM-stage may be somewhat confusing.
One might think that this staging is largely based on the findings of physical examination, but it is mostly based on the results of imaging and image-guided biopsy.
Neoadjuvant chemotherapy
Neoadjuvant chemotherapy (from neo: before and adjuvare: to aid) is given to patients with locoregionally advanced cancer to decrease the tumor burden prior to surgery.
In patients with localized cancer in whom there is an indication for adjuvant therapy (see table under adjuvant therapy), the therapy may be given before surgery (neo-adjuvant) or after surgery (adjuvant).
Sentinel Node
In patients who have no detectable axillary lymph node metastases with ultrasound-guided fine needle aspiration (FNA), a sentinel node procedure is performed in order to acquire information about the lymph node status of the axilla.
Breast Conserving Therapy or Mastectomy
BCT is performed when excision is possible with good cosmetic result. Surgery is always followed by radiotherapy.
Good cosmetic result depends on the size of the tumor and the size of the breast.
Nowadays it is possible to treat larger tumors with BCT by applying plastic- and reconstructive surgery with fat grafting and flap-procedures.
If BCT is not possible - or wanted by the patient - a mastectomy is performed.
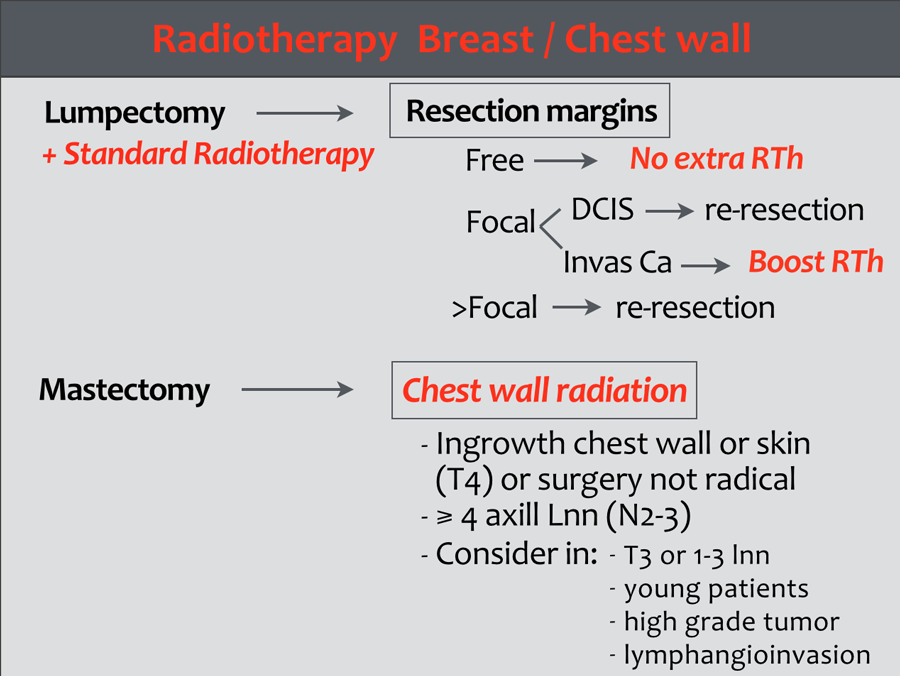
Radiotherapy
Treatment is planned on the final stage and tumor characteristics.
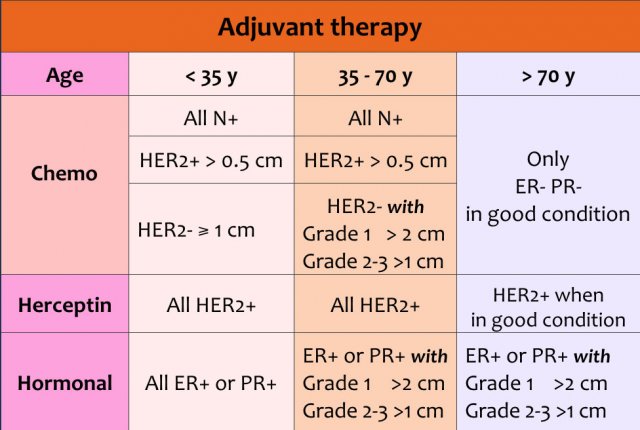
Adjuvant systemic chemotherapy and endocrine therapy are complementary treatment options after primary locoregional treatment. These are aimed at eliminating any distant occult metastases which may be present, but cannot be detected yet.
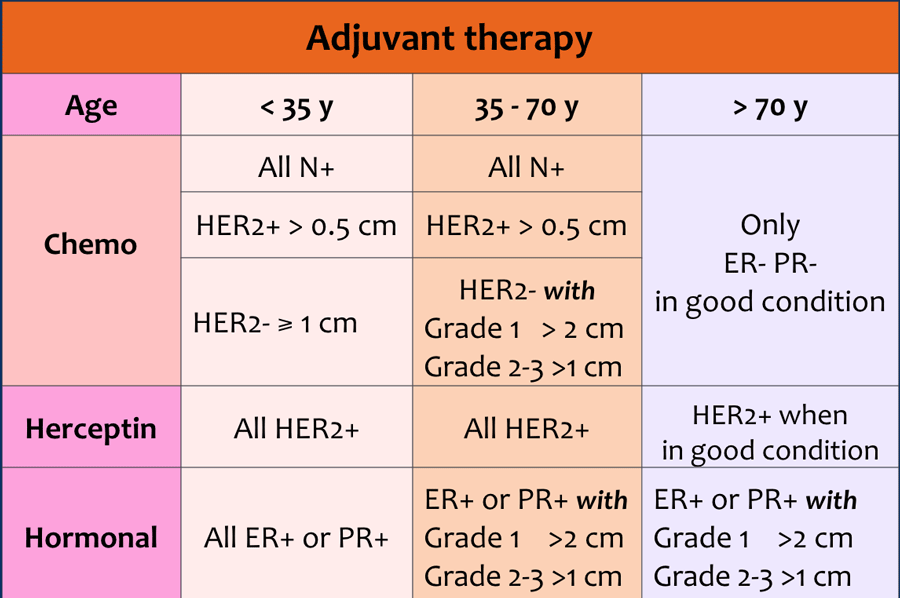
Adjuvant therapy can consist of chemotherapy and/or herceptin and/or hormonal therapy.
The choice of therapy depends on:
- Age of the patient
- Size of the tumor
- Lymph node status
- Grade of the tumor
- Receptors for estrogen, progesteron and HER 2-neu
DCIS
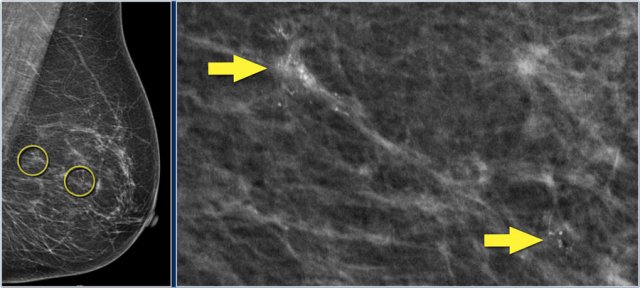
 There are multiple fine, linear calcifications in a large segment of the breast. These are suspicious calcifications and are typically seen in DCIS. Click to enlarge
There are multiple fine, linear calcifications in a large segment of the breast. These are suspicious calcifications and are typically seen in DCIS. Click to enlarge
Ductal carcinoma in situ is the most common non-invasive breast cancer.
In situ means 'in place' and refers to the fact that the cancer has not moved out of the duct and does not infiltrate the surrounding tissue.
DCIS can progress to become invasive cancer, but likelihood estimates vary.
80% of DCIS is diagnosed on mammography alone on the basis of microcalcifications (figure), followed by vacuum-assisted biopsy. Very few cases of DCIS present as a palpable mass.
The problem with DCIS is:
- The size of DCIS is determined by the size of the area with microcalcifications, but this often is an underestimation. As a result of this, the re-resection rate in DCIS is higher than in invasive cancer without DCIS.
- In 15-20% of patients initially diagnosed with DCIS, an invasive cancer is found by the pathologist in the resected specimen. This occurs more often in DCIS grade II and III. These patients will have to be treated as having invasive cancer.
Because of these problems, a pre-operative MRI is performed in cases with high-grade DCIS in some institutions, to better delineate the spread of the tumor and to assess if there are any signs of invasive growth.
Whether this is of benefit to the patient is still unclear.
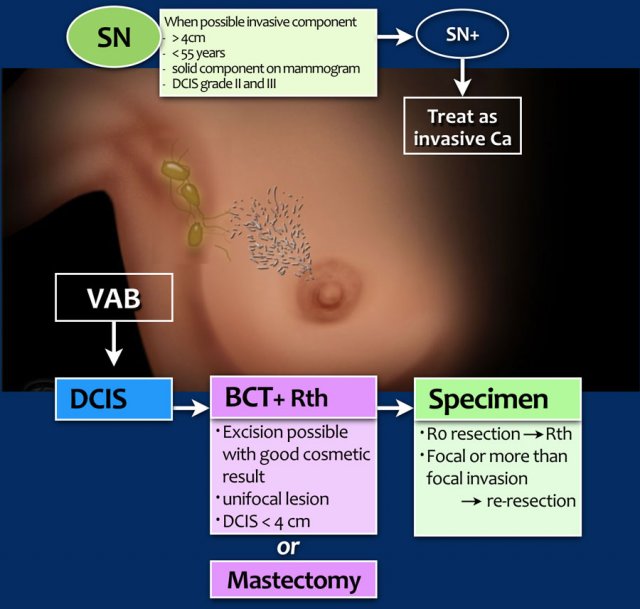
Treatment of DCIS
consists of breast conserving therapy (BCT) or mastectomy depending on whether excision is possible with good cosmetic result, and of course, on patient preference.
BCT
consists of microscopically complete tumor excision and radiotherapy. A radiation boost may be considered, particularly in younger patients.
Sentinel node procedure
should be considered in patients likely to have additional invasive carcinoma (see table).
Specimen
If the DCIS is completely resected, this is called an R0-resection.
After breast conserving surgery, these patients are treated with radiotherapy.
If there is focal invasion of the resection margins, an extra boost is given.
If there is more than focal invasion (> 4mm) then a re-resection or mastectomy will be performed.
Study the images and then continue reading.
Click image to enlarge.
The findings are:
- Multiple suspicious calcifications in a large segmental area of the breast.
- Multiple clusters - many with a linear branching arrangement - typical for DCIS.
Because of the large area with calcifications, a mastectomy was planned.
A sentinel node procedure was also performed because of the estimated size of the DCIS.
Whenever a mastectomy is planned, an SN-procedure is routinely included, because it cannot be performed afterwards.
Pathology showed small foci of invasive cancer.
In this patient, there are two groups of suspicious calcifications which are about 5 cm apart.
First the larger group was biopsied.
The idea was that if these calcifications were benign, then the smaller cluster would probably also be benign.
However, the larger group of calcifications turned out to be DCIS grade 2.
So a second biopsy was needed of the smaller group of calcifications, and this also turned out to be DCIS grade 2.
A mastectomy with a sentinel node procedure was planned.
The total area of DCIS was thought to be too large for breast conserving treatment.
Localized breast cancer
Localized or early stage breast cancer includes:
- T1-2: tumors < 5 cm
- N0-1 : no nodes or involvement of <4 axillary nodes
Axilla
The axilla is initially examined with ultrasound. When pathologic lymph nodes are detected, the next step is FNA.
If the results are positive (N+), usually a PET-CT is performed to exclude more advanced disease.
When no suspicious lymph nodes are found with ultrasound (N), the next step is a sentinel node procedure.
BCT in Localized breast cancer
When breast conserving surgery is performed, there is always a risk of leaving small areas of tumor behind - even when the resection margins are tumor-free.
This is the main reason why in BCT, surgery is always combined with radiotherapy.
The risk of local recurrence is greater in tumors surrounded by an extensive DCIS component.
Depending on the receptor status of the tumor, most patients with localized cancer will also receive adjuvant or neoadjuvant treatment.
Only older patients (>70y) or patients younger than 70 years with small, low-grade tumors do not need chemotherapy (see table).
No further investigations are necessary in localized breast cancer, because the chance of finding metastatic disease is very low.
Locoregional advanced cancer
Locally advanced breast cancer includes:
- T3: tumors > 5cm
- T4: tumors with invasion of the skin or chest wall
- N2-3: ⩾ 4 axillary lymph nodes or ipsilateral supraclavicular, infraclavicular or internal mammary nodal involvement
Treatment
In the past these tumors were synonymous with inoperable breast cancer, but this has changed after the introduction of neoadjuvant therapy.
These patients are first treated with neoadjuvant chemo- and/or hormonal therapy. If there is a good response to the neoadjuvant therapy, the next step is surgery and sometimes even breast-conserving surgery is possible.
Before neoadjuvant therapy is given, a clip is placed in the tumor. Sometimes the response is so good that no residual tumor can be found.
There is current debate whether these patients still need surgery, but currently, most of these patients are treated with BCT (lumpectomy centered on the clip, followed by radiotherapy ).
Surgery and other procedures
FNA of axillary lymph nodes
The criteria for performing Ultrasound guided FNA or biopsy of an axillary lymph node are:
- Cortex > 2.3 mm, not measured at the poles of the node.
- Disappearance of fatty hilum.
- Asymmetric bulging of the cortex.
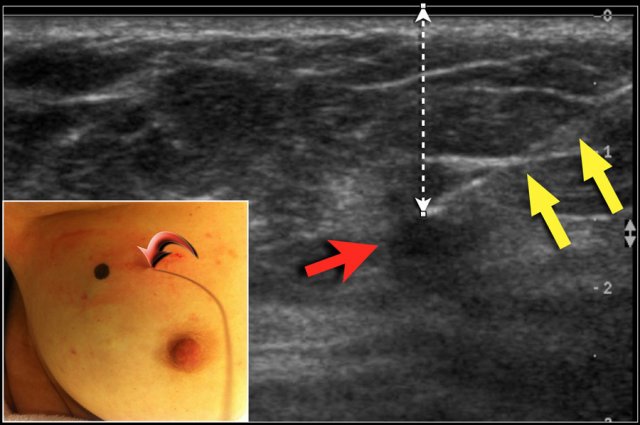
Wire localisation
DCIS and many small tumors are not palpable. In these cases, the radiologist places a hook wire in the area that needs to be removed.
The wire is placed within the tumor or the area of DCIS, either US-guided or stereotactically.
With US, the position of the tumor is marked on the skin, while the patient holds her arm in the same position as during surgery. The exact depth of the tumor beneath the skin is also noted.
The figure shows the tract of the wire (yellow arrows) towards the tumor (red arrow). Notice the black dot on the skin to mark the exact position of the tumor.
The curved arrow indicates the entrance of the wire into the breast.
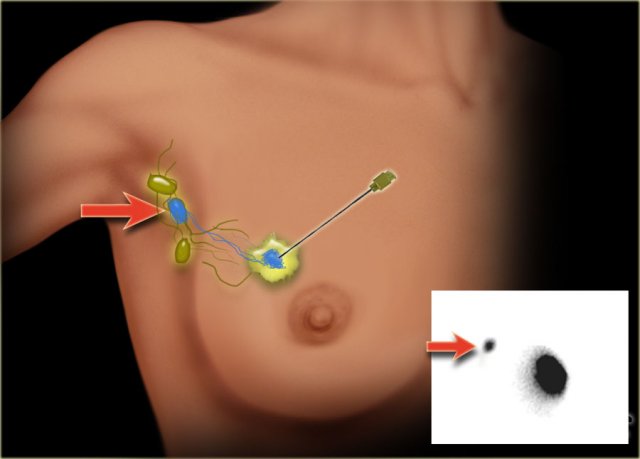
Sentinel node
The sentinel node procedure (SLN) has become the standard method for staging the axilla in breast cancer patients with a negative axillary ultrasound (cN-).
SLN biopsy provides accurate staging information while avoiding the morbidity of a complete axillary lymph node dissection.
A radiopharmacon together with a dye is injected in or near the tumor (figure). These tracers flow through the lymph ducts to the lymph nodes.
The first lymph node(s) to receive the tracers are removed.
Usually about three nodes are removed. Removal of more than three SLNs does not increase the accuracy of finding a positive node.
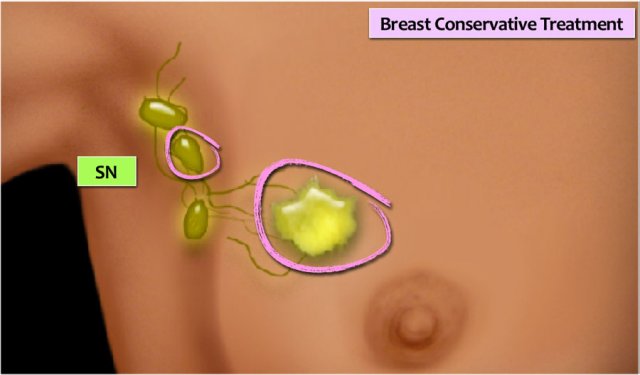
BCT
Breast Conserving Treatment (BCT) is also known as lumpectomy or wide excision. A locoregional excision of the tumor is combined with a sentinel node procedure for axillary node staging, followed by radiotherapy of the breast with or without a boost.
MRI-compatible clips should be placed in the tumor bed for the purpose of obtaining accuracy in radiotherapy.
Re-excision is indicated if there is more than a focal tumor-positive resection margin (see pathology).
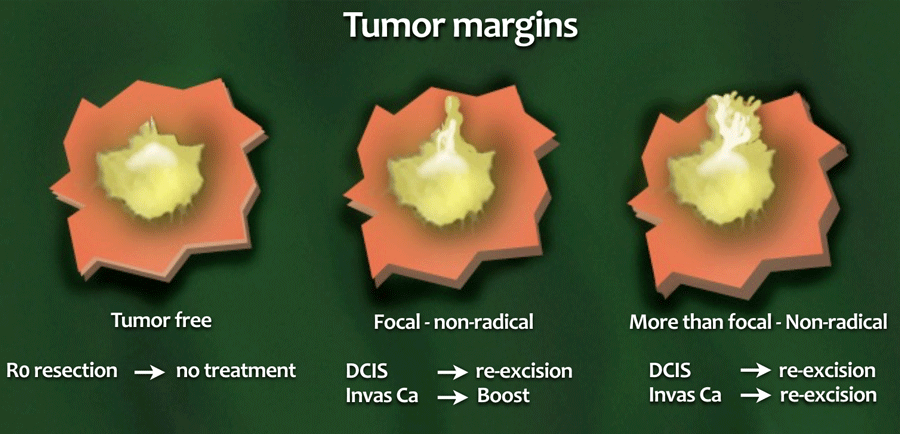
Resection margins in BCT
- R0-resection
No tumor in the resection margin. - Focal non-radical
Less than 4mm of the resection margin contains tumor. - More than focal
More than 4mm of the margin is involved.
Invasive cancer
Re-excision is indicated if there is a more than focal tumor-positive resection margin, as this is the most important risk factor for local recurrence.
When the resection margin is only focally involved, adjusting the radiation dose appears to be a good alternative to re-excision.
DCIS
Treatment is more aggressive in DCIS, since re-excision or mastectomy is advocated in any involvement of the resection margins.
Mastectomy
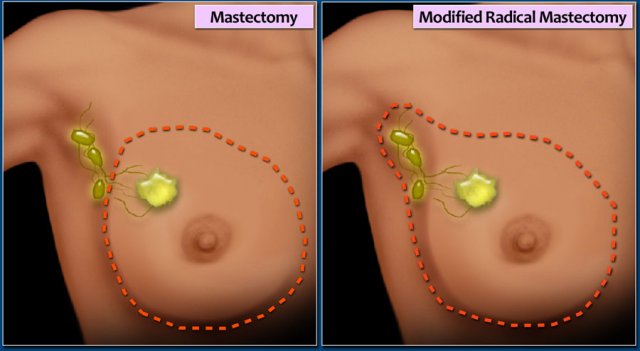
Simple Mastectomy
Removal of the whole breast. Some of the axillary lymph nodes (level I) may also be removed.
Modified radical mastectomy
The breast, most or all of the axillary lymph nodes, and the fascia of the chest wall muscles is removed.
The pectoralis muscle is not removed.
Nipple sparing mastectomy
In most cases the nipple is removed in a mastectomy.
The nipple can be spared if:
- Tumor is > 2 cm away from the nipple
- Tumor size < 5 cm
- No multifocallity
- No positive axillary lymph nodes
Oncoplastic surgery
Direct reconstruction
Direct breast reconstruction using implants.
DIEP-flap
The deep inferior epigastric artery perforator (DIEP) flap is a common technique in which skin and subcutaneous fat are taken from the abdomen to recreate the breast.
SGAP flap
The superior gluteal artery perforator (SGAP) flap uses tissue from the top of the buttocks to create breast tissue. This is usually done if patients do not have adequate skin and tissue in their abdomens or have had previous abdominal surgeries.
TRAM flap
In a pedicle TRAM flap, the tissue remains attached to its original site, retaining its blood supply.
The flap, consisting of the skin, fat, and muscle with its blood supply, is tunneled beneath the skin to the chest.
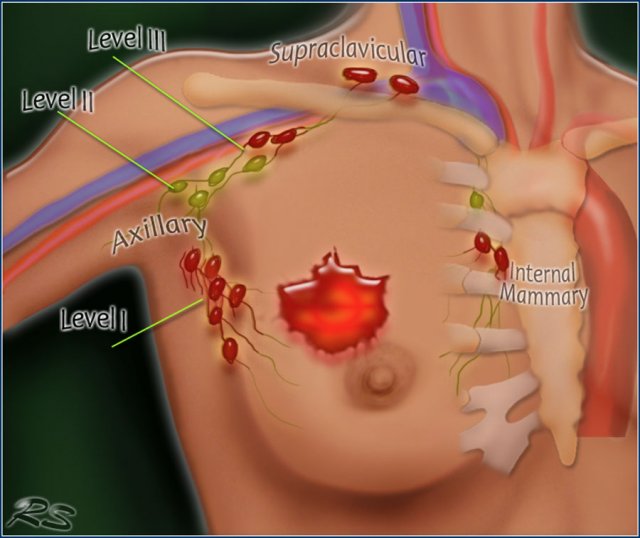
Axillary Lymph Node Dissection
In Axillary Lymph Node Dissection (ALND) the axillary lymph nodes are removed.
There are three levels of axillary lymph nodes:
Level I is below the lower edge of the pectoralis minor muscle.
Level II is at the level of the pectoralis minor.
Level III is above the pectoralis minor.
In ALND levels I and II are removed.
Nowadays, surgeons try to avoid an ALND by performing a sentinel node dissection or by downstaging the axilla with neoadjuvant therapy.
ALND is performed when there are many positive nodes or when neoadjuvant therapy is not possible.
MARI procedure
The MARI procedure is a new minimal invasive method to assess the pathological response of an axillary lymph node metastasis after neoadjuvant systemic treatment.
The tumor-positive axillary lymph node is marked with a radioactive iodine (I) seed.
This marked lymph node is the so-called MARI-node.
After neoadjuvant treatment, the MARI node is selectively removed using a γ-detection probe.
If the node has become tumor-negative and the sentinel node is also negative, no additional treatment of the axilla is necessary.
This method allows axilla-conserving surgery in patients who respond well to neoadjuvant treatment.
Pathology
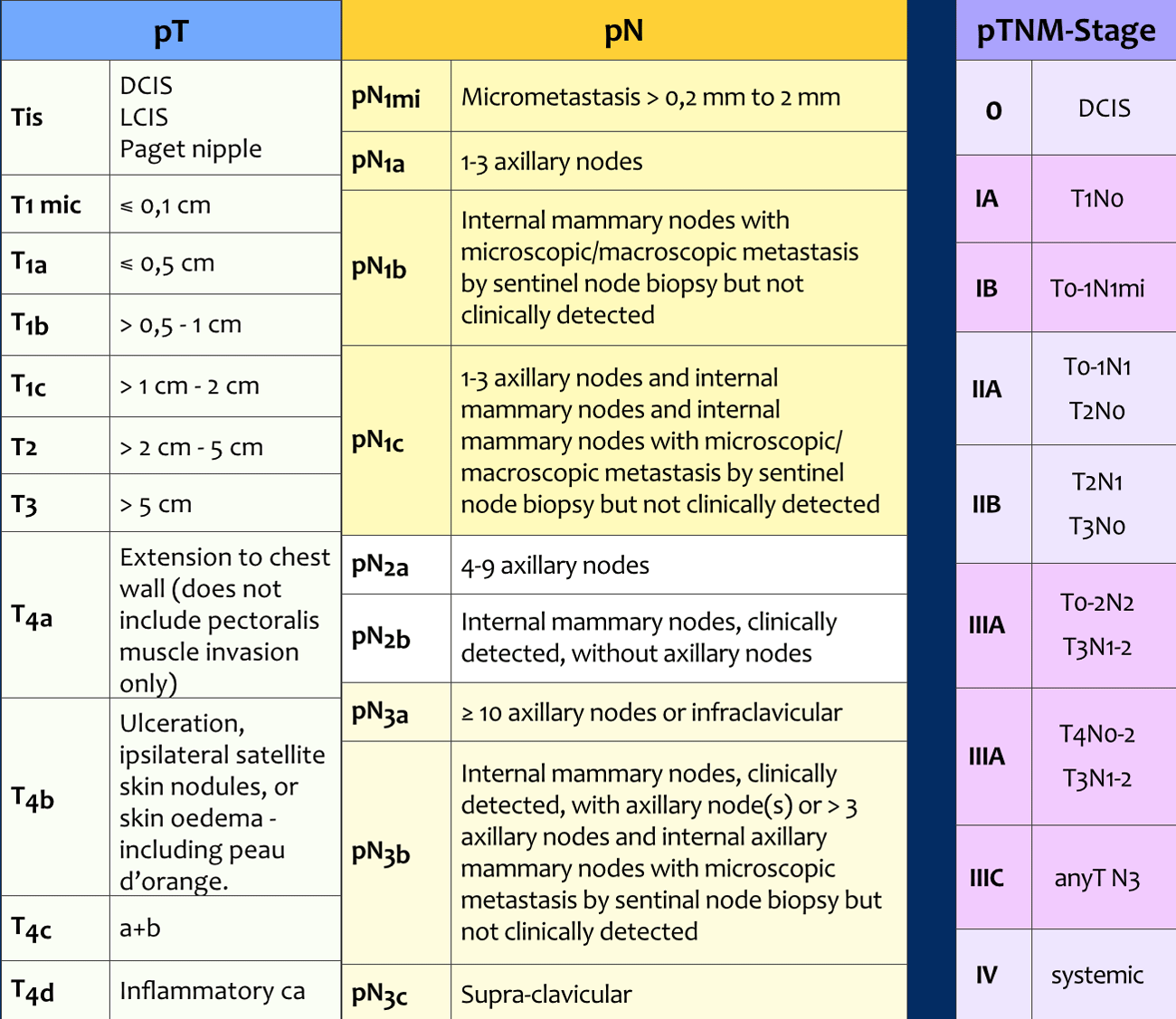
pTNM-stage
Following surgery, the surgical specimens of the tumor, the sentinel node or axillary nodes after dissection are analyzed by the pathologist. This results in a pTNM-stage (table).
The pTNM may differ from the cTNM-stage, and sometimes the original treatment plan has to be adjusted.
For example, if the margins of a resected specimen are not tumor-free, re-excision or adjustment of radiotherapy may be necessary.
For a proper pathological pT-classification there can be no gross tumor in the resection margins.
Classification is possible with microscopic tumor in the margins.
The tumor size includes only the invasive component.
If there is a large in situ component (e.g. 4 cm) and a small invasive component (e.g. 0,5 cm), the tumor is measured 0,5 cm and coded pT1a.
The analysis of the surgical specimen of the tumor, the sentinel node or axillary nodes after dissection by the pathologist results in a pTNM or ypTNM-stage.
Other tumor characteristics such as tumor-grade, hormone receptors, lymphangio-invasion and gene-expression are also examined, as these characteristics also determine the risk of local recurrence and systemic disease.
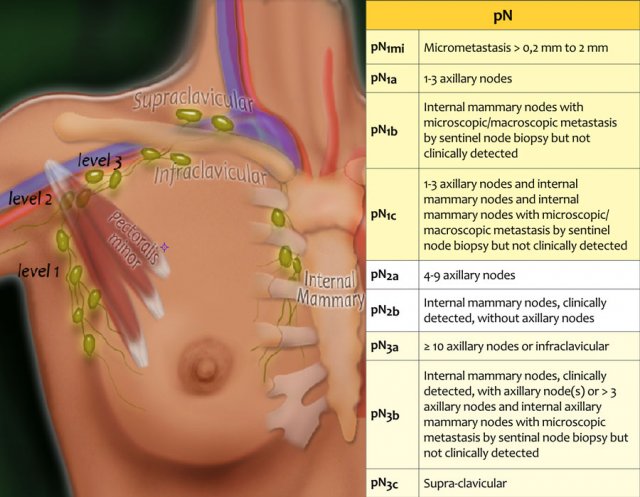
pN-stage
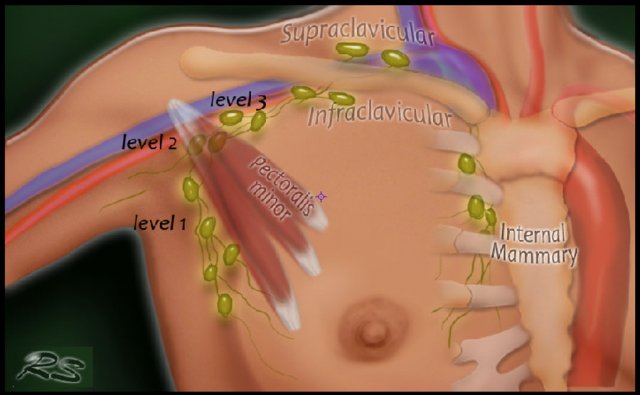
Seventy-five percent of the lymphatic drainage of the breast is to the axillary nodes.
Level 1 nodes are located below the lower edge of the pectoralis minor muscle.
Level 2 nodes are located at the level of the pectoralis minor muscle.
Level 3 are above the pectoralis minor muscle and drain to the infra- and supraclavicular nodes.
The minority of the lymphatics drain to the internal mammary nodes, or less commonly, via a transpectoral route.
ypN-stage
In patients who have received neoadjuvant chemotherapy, the lymph node status will be affected.
For this reason, a y is added to the pN-stage. For instance, if a sentinel node procedure is performed after neoadjuvant therapy and 3 positive nodes are found at pathology, this will result in a ypN1a.
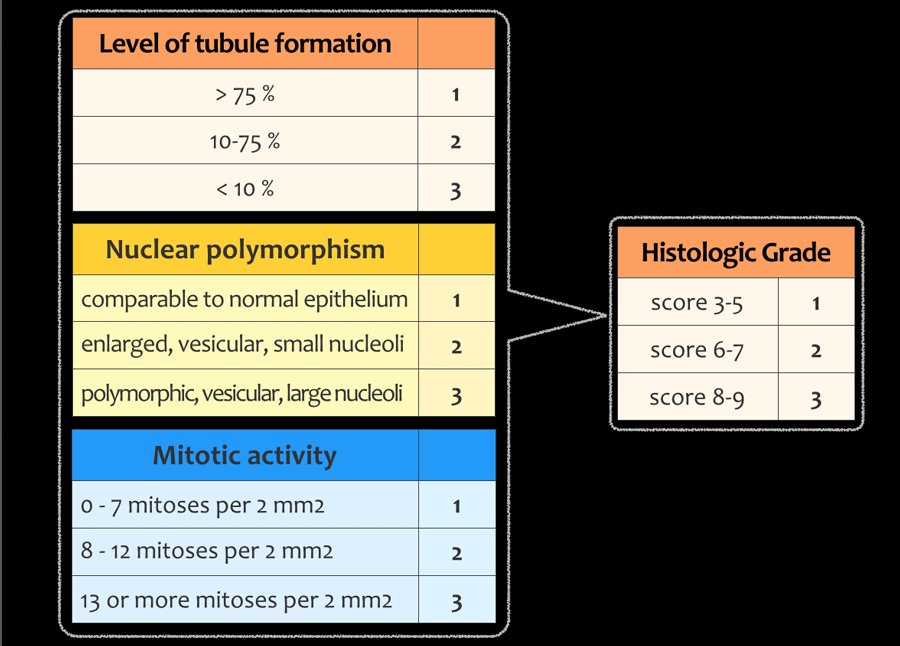
Tumor-grading
The histological grade of a tumor is another important prognostic factor in the staging of a tumor.
A high-grade tumor is more likely to spread, necessitating more aggressive adjuvant therapy.
Tumor grading is done according to the modified Bloom and Richardson guidelines and is based on the total score of tubule formation, nuclear polymorphism and mitotic activity.
Gene expression
The prognosis and treatment of patients with breast cancer depends on many factors:
- TNM-stage.
- Tumor grade
- Lymphangio-invasion
- Hormone receptors
- Gene expression of the tumor
Analysis of the DNA of the tumor and the gene expression has led to the identification of subtypes of breast cancer that have distinct clinical outcomes and different responses to chemotherapy.
It can help to select patients that need to be treated aggressively and who don't.
Gene expression is a process in which a gene gets turned on in a cell to make RNA and proteins.
Some of these proteins, such as HER2/neu, stimulate the growth of tumor cells.
HER2/neu
This protein is produced in abnormally high amounts in about 20% of breast tumors. Breast cancers that overproduce HER2 tend to be more aggressive and are more likely to recur.
Trastuzumab (Herceptin) targets the HER2 protein specifically, and in conjunction with adjuvant chemotherapy, can lower the risk of recurrence by about 50% when compared with chemotherapy alone.
Whenever breast cancer recurs or spreads, the cancer cells should be retested for HER2 as well as for hormone receptor status, as these may differ from the original cancer in up to 20 to 30 percent of cases (8).
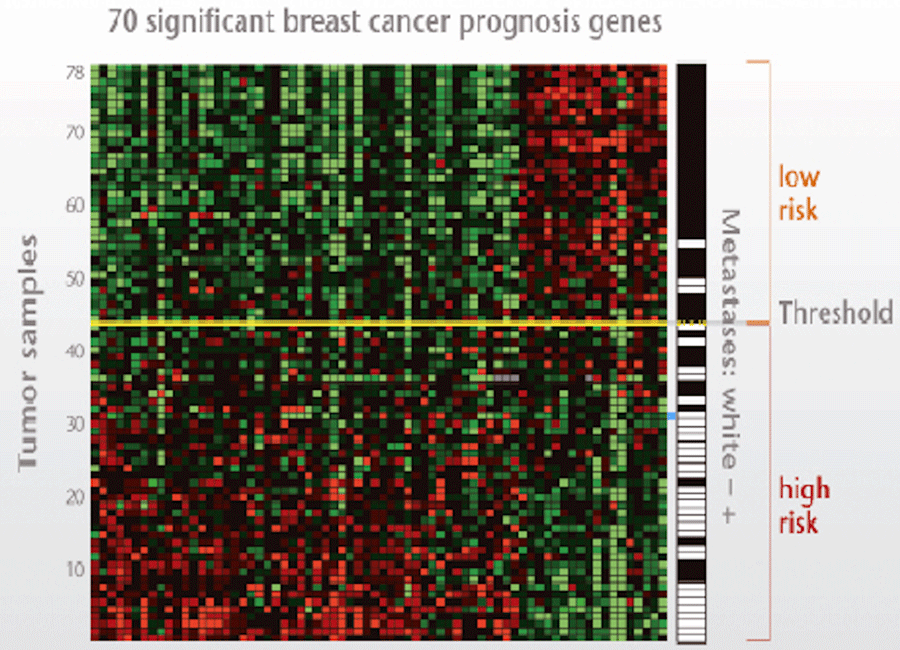
Mammaprint
On the basis of patterns of gene expression, breast cancer may be subdivided into different molecular subtypes or profiles.
One of these profiles is the MammaPrint®, which tests for 70 different tumor genes and divides patients into a high- and low-risk category and whether patients will benefit from chemotherapy or not.
Systemic Therapy
Adjuvant systemic therapy
Adjuvant systemic chemotherapy and endocrine therapy are administered as complements to primary locoregional treatment with the aim of eliminating any distant occult metastases (which may be present, but cannot be detected yet).
Adjuvant therapy can consist of chemotherapy, herceptin and/or hormonal therapy.
Herceptin as added to chemotherapy in patients who are HER2/neu positive and hormonal therapy is given to patients who have estrogen or progesteron receptors (ER+ or PR+)
In older women who are ER+ or PR+ sometimes only hormonal therapy ois given in trying to stabalize the disease.
Neoadjuvant therapy
Neoadjuvant chemotherapy or primary systemic treatment used to be given exclusively to patients with locally advanced disease, i.e. stage III and stage II-patients with a large tumor (T3N0).
Current policy is that more and more patients with early cancer, who have an indication for chemotherapy, are also being treated with neoadjuvant chemotherapy instead of adjuvant therapy post-surgery.
Advantages of this policy is that neoadjuvant therapy allows for monitoring of the response to treatment, so that the type of chemotherapy can be adjusted when there is no adequate response.
Another advantage is that the size of the lumpectomy can be reduced, and breast conserving treatment may be possible in patients who would otherwise have been treated with mastectomy.
Even in clinical complete remission, a combination of surgery and radiotherapy was found necessary to avoid local recurrence.
Preconditions for starting neoadjuvant therapy
Breast diagnostics
- Histological biopsy: determination of the tumor grade, hormone receptors and HER-2-amplification.
- Accurate documentation of the initial tumor size and metastases by means of MRI, unless it can be reliably determined using mammography and ultrasound.
- Photograph cT4 tumors in order to record skin metastases.
- Placing a radio-opaque marker or a radioactive iodine-125 seed in the tumor, independent of whether mastectomy or BCT is chosen.
Regional diagnostics
- Recording axillary lymph node status clinically and with ultrasound.
- If cN1-3: cytological confirmation. Mark the positive lymph node with a marker or place a radioactive iodine seed for axillary staging after neoadjuvant systemic treatment (MARI procedure).
- if cN0: SWK procedure. Some prefer prior to neoadjuvant treatment, others post treatment.
Screening for distant metastasis
- Indicated in stage III breast cancer
- Consider for stage II clinical N+ breast cancer
Neoadjuvant chemotherapy
The response rate to neoadjuvant chemotherapy is 80-90%.
The risk of progression is less than 5-10%. There is no clear treatment plan in case of progression, which is defined as RECIST > 20% increase in diameter during chemotherapy.
Neoadjuvant hormonal therapy
There are no randomized studies available comparing neoadjuvant hormonal therapy with the same treatment postoperatively. Similar to chemotherapy, neoadjuvant hormonal therapy appears to make downstaging possible for hormone receptor-positive tumors, with an improved chance of radical surgery in stage III or BCT when mastectomy initially seemed necessary.
Neoadjuvant trastuzumab
The addition of trastuzumab to neoadjuvant chemotherapy increases the percentage of pathological complete response.
Radiotherapy
Radiotherapy of the breast
Radiotherapy forms an integral part of breast conserving treatment in breast cancer. Most recurrences after BCT are due to growth of residual tumor.
The whole breast is irradiated with an optional boost dose to the tumor bed.
Patients with a small and low grade DCIS have a low risk of recurrence and additional radiotherapy may not be justified (15).
Radiotherapy of the chest wall
Indications for radiotherapy of the chest wall following mastectomy are:
- Tumor-positive resection margin of the primary tumor.
- T4 tumor
- pT3, depending on one or more of the following risk factors: angio-invasive growth, grade 3 tumor, and/or age ≤ 40 years
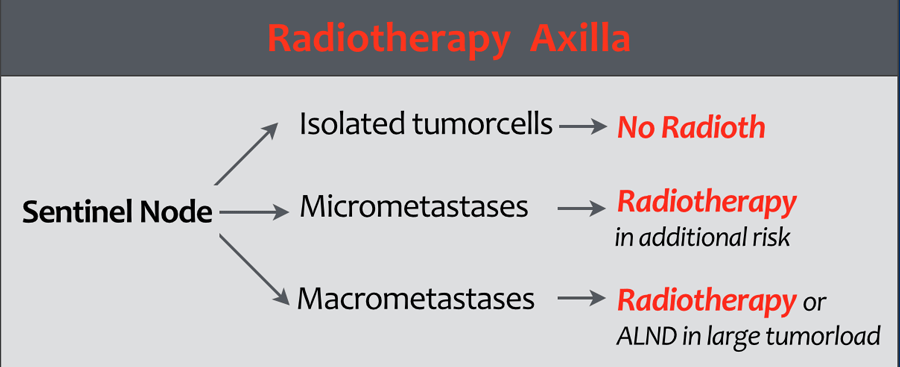
Radiotherapy of the axilla
Axillary radiotherapy is a safe alternative to axillary lymph node surgery and has a less risk of lymphedema.
There is a lot of discussion concerning the indications for radiotherapy of the axilla.
Many guidelines are not evidence-based.
Approaches differ between countries and between institutions.
In the Duthc Breast Cancer Guideline 2012 the indications are as follows (1):
- Isolated tumor cells in the sentinel node:
Radiotherapy is not recommended. - Micrometastases in the SN: Radiotherapy is only recommended in case of additional risk factors.
- Macrometastases:
Radiotherapy is indicated.
In cases with a large axillary tumor load an axillary lymph node dissection is recommended.
These guidelines however do not apply to other countries and are also not evidence-based, but expert opinions.
You must reach a consensus for radiotherapy indications in your own institution.
Special types of breast cancer
Triple negative breast cancer
Approximately 15-20% of breast cancers are so-called triple negative and characterized by the absence of ER-, PR- and HER2-overexpression.
These tumors occur more often at a young age, are high-grade, and on presentation often substantial in size with metastases to the axillary lymph nodes.
Triple negative tumors have a poorer prognosis with rapid recurrences, and frequent brain metastases.
Studies have found that these tumors respond better to standard neoadjuvant chemotherapy with anthracyclines and taxanes compared to other tumor types.
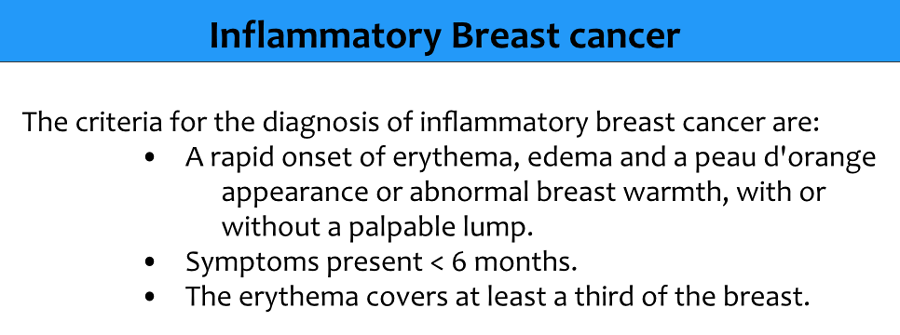
Inflammatory carcinoma
Inflammatory carcinoma or mastitis carcinomatosa is a separate category of breast cancer and accounts for 1-5% of all breast cancers.
It is characterized by diffuse redness, peau d'orange and swelling of the breast, therefore it is mainly a clinical diagnosis.
It is a rare and very aggressive disease in which tumor emboli block the lymphatics in the skin.
The TNM classification is T4D (stage III).
Dimpling of the skin and nipple retraction is not the same as inflammatory breast cancer and may occur in any T-stage without affecting the classification.
Paget disease of the nipple
The diagnosis of Paget disease of the nipple can only be made if there is no disease in the underlying breast parenchyma.
Carcinomas in the breast parenchyma associated with Paget disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget disease should still be noted.
Lobular carcinoma
Invasive lobular carcinoma is the second most common type of breast cancer after invasive ductal carcinoma (17).
Invasive lobular carcinomas tend to be more difficult to detect on mammography, because instead of forming a lump, the cancer cells typically spread to the surrounding connective tissue in a line formation.
Additional Imaging
PET-CT
When to use PET-CT
PET-CT is recommended in patients with locoregional disease to search for distant metastases. PET-CT has a higher sensitivity and specificity for the detection of distant metastases and locoregional recurrence than conventional imaging.
The sensitivity of PET-CT however is too low for the detection of a primary breast cancer and PET-CT also does not play a role in staging of a clinically negative axilla and cannot replace the SN-procedure.
However, when axillary lymph node metastases are detected by FNA or sentinel node procedure, and the PET-CT shows uptake in lymph nodes, then all these nodes are regarded as positive, because the positive predictive value is high.
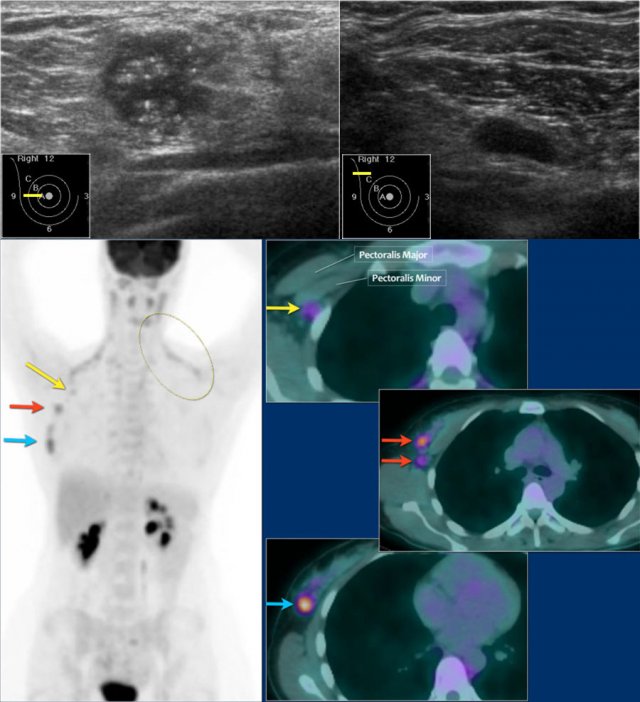
Here an example of a patient with advanced breast cancer.
The US of the breast showed an 18mm echopoor lesion with irregular margins and microcalcifications.
US of the axilla revealed a lymph node with no fatty hilum.
FNA was performed, and both the tumor and the lymph node were positive for adenocarcinoma.
The lesion was clinically staged as cT1N+. A PET-CT was performed.
On the upper PET-CT image a level II node is positive just underneath the pectoralis minor (yellow arrow).
Multiple axillary nodes were found but no systemic metastases.
Normal brown fat is seen along the neck and shoulder muscles on both sides (circle).
This patient was planned for neoadjuvant therapy and biopsies were taken for the determination of the tumor grade, hormone receptors and HER-2-neu-amplification.
Here another PET-CT example.
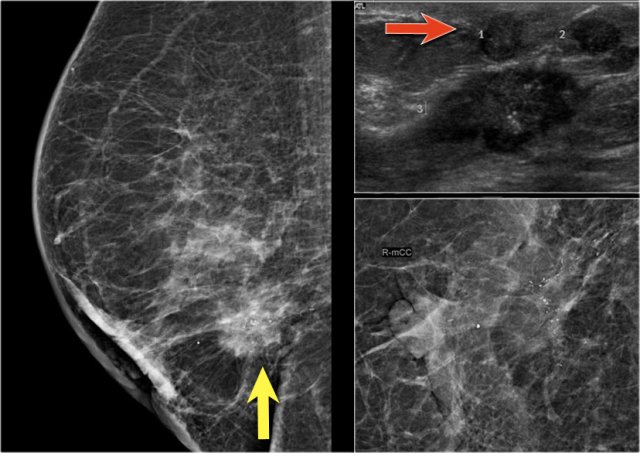
On the mammogram, there is a tumor with secondary skin retraction.
Notice multiple linear calcifications within the tumor - click on image to enlarge. This means that the infiltrating tumor developed within an area of ductal carcinoma in situ or DCIS.
Ultrasound demonstrates tumorfoci within the skin, i.e. T4b (red arrow).
Continue...
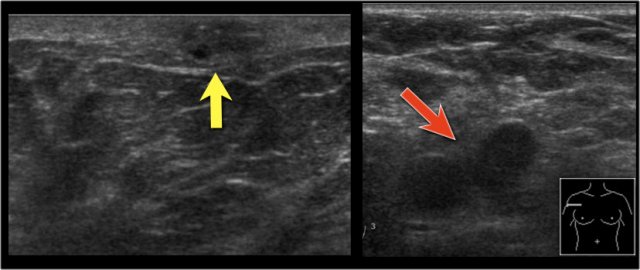
On this ultrasound image, there is another satellite nodule within the skin (yellow arrow).
Multiple enlarged axillary nodes are seen (red arrow).
Fine needle aspiration demonstrated metastases within these nodes.
This tumor was staged as T4bN+. This means that we are dealing with locoregionally advanced disease.
These patients are at risk for systemic disease and additional imaging was performed.
Continue...
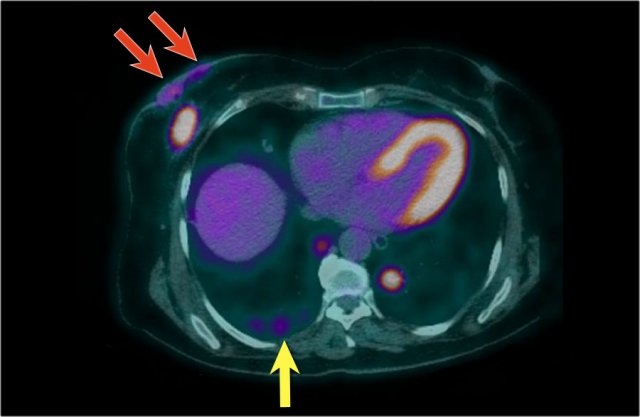
Here we see the PET-CT image of this patient. Both the breast carcinoma as well as the spread to the skin are demonstrated (red arrows). There are multiple metastases in both lungs - a metastasis in the right lung is indicated by yellow arrow; notice the larger metastasis in the left lung.
In addition, there were also bone- and liver metastases and multiple axillary lymph nodes were seen (not shown on this image).
The cTNM-stage of the tumor is T4bN3M1.
MRI
MRI is found to be better at indicating the size of DCIS and invasive cancer and is also better in detecting multifocal or contralateral disease. There is still debate concerning the role of preoperative MRI in patients with breast cancer, because overestimation occurs due to the presence of enhancing benign proliferative disorders.
Many do not recommend a preoperative MRI, since it has not resulted in a better outcome or significantly lower percentage of reoperations.
It is only recommended in high grade DCIS with indeterminate tumor size in patients who would like to be eligible for BCT.
MRI however plays an important role in diagnostic problem solving and in the screening of high-risk patients.
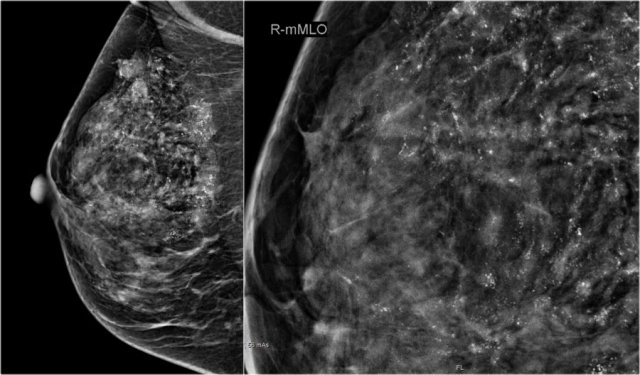
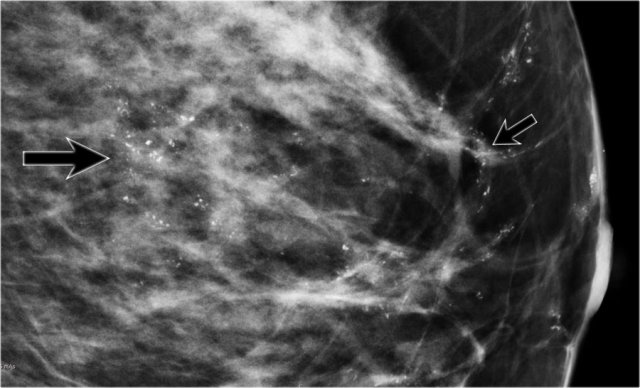
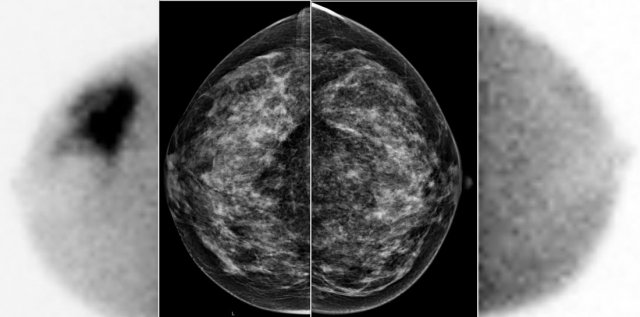 A large tumor in the right breast is easily detected with BSGI, while hardly visible on the mammogram.
A large tumor in the right breast is easily detected with BSGI, while hardly visible on the mammogram.
BSGI
Breast-specific gamma imaging (BSGI) is an adjunct modality for breast imaging which, like MRI, uses a physiologic approach to identify lesions in the breast.
The sensitivity and specificity are comparable to MRI, and are much higher than for mammography - especially in dense breast tissue.
The higher radiation dose compared to mammography makes BSGI unsuitable for screening, but it is an excellent tool for problem-solving.
Just like MRI, it is good at detecting multifocal or contralateral disease.